p110δ PI3 kinase pathway: emerging roles in cancer
- PMID: 23459844
- PMCID: PMC3585436
- DOI: 10.3389/fonc.2013.00040
p110δ PI3 kinase pathway: emerging roles in cancer
Abstract
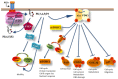
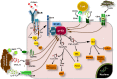
Class IA PI3Ks consists of three isoforms of the p110 catalytic subunit designated p110α, p110β, and p110δ which are encoded by three separate genes. Gain-of-function mutations on PIK3CA gene encoding for p110α isoform have been detected in a wide variety of human cancers whereas no somatic mutations of genes encoding for p110β or p110δ have been reported. Unlike p110α and p110β which are ubiquitously expressed, p110δ is highly enriched in leukocytes and thus the p110δ PI3K pathway has attracted more attention for its involvement in immune disorders. However, findings have been accumulated showing that the p110δ PI3K plays a seminal role in the development and progression of some hematologic malignancies. A wealth of knowledge has come from studies showing the central role of p110δ PI3K in B-cell functions and B-cell malignancies. Further data have documented that wild-type p110δ becomes oncogenic when overexpressed in cell culture models and that p110δ is the predominant isoform expressed in some human solid tumor cells playing a prominent role in these cells. Genetic inactivation of p110δ in mice models and highly-selective inhibitors of p110δ have demonstrated an important role of this isoform in differentiation, growth, survival, motility, and morphology with the inositol phosphatase PTEN to play a critical role in p110δ signaling. In this review, we summarize our understanding of the p110δ PI3K signaling pathway in hematopoietic cells and malignancies, we highlight the evidence showing the oncogenic potential of p110δ in cells of non-hematopoietic origin and we discuss perspectives for potential novel roles of p110δ PI3K in cancer.
Keywords: PTEN; cancer; hematologic malignancies; p110δ PI3K; solid tumors.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

