Characteristics of patients preferring once-daily controller therapy for asthma and COPD: a retrospective cohort study
- PMID: 23460035
- PMCID: PMC6442780
- DOI: 10.4104/pcrj.2013.00017
Characteristics of patients preferring once-daily controller therapy for asthma and COPD: a retrospective cohort study
Abstract
Background: Patient preference is an important factor when choosing an inhaler device for asthma or chronic obstructive pulmonary disease (COPD).
Aims: To identify characteristics of patients with asthma or COPD who prefer a once-daily controller medication regimen.
Methods: This retrospective observational study used electronic patient records and linked outcomes from patient-completed questionnaires in a primary care database. We compared the characteristics of patients indicating a preference for once-daily therapy with those who were unsure or indicating no preference.
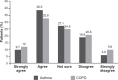
Results: Of 3,731 patients with asthma, 2,174 (58%) were women; the mean age was 46 years (range 2-94). Of 2,138 patients with COPD, 980 (46%) were women; the mean age was 70 years (range 35-98). Approximately half of the patients in each cohort indicated once-daily preference, one-quarter were unsure, and one-quarter did not prefer once-daily therapy. In patients with asthma or COPD, the preference for once-daily controller medication was significantly associated with poor adherence and higher concerns about medication. In asthma, good control and low self-perceived controller medication need were associated with once-daily preference. By contrast, in COPD, a high self-perceived need for controller medication was associated with once-daily preference. There was no significant relationship between once-daily preference and age, sex, disease severity, or exacerbation history.
Conclusions: Understanding patient preferences may help prescribers to individualise therapy better for asthma and COPD.
Conflict of interest statement
DP has consultant arrangements with Almirral, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Medapharma, Novartis, Napp, Nycomed, Pfizer, Sandoz, and Teva. He or his research team have received grants and support for research in respiratory disease from the following organisations in the last 5 years: UK National Health Service, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer, and Teva. He has spoken for Almirral, AstraZeneca, Activaero, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Novartis, Merck, Mundipharma, Pfizer, and Teva. He has shares in AKL Ltd which produces phytopharmaceuticals. He is the sole owner of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care. AJL receives ad hoc payment for statistical consultancy with RIRL. EJS and JvZ are employees of RiRL. EVH is a consultant to RiRL and has done freelance writing work for Merck, Aerocrine, and TevaFrance. At the time this study was conducted, LK and AC were employees of RiRL and AW was an employee of GSK R&D.
Figures
Comment in
-
Is once enough? Understanding the preferences of COPD and asthma patients for once- versus twice-daily treatment.Prim Care Respir J. 2013 Jun;22(2):140-2. doi: 10.4104/pcrj.2013.00053. Prim Care Respir J. 2013. PMID: 23708114 Free PMC article. No abstract available.
References
-
- World Health Organization. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization, 2003.
-
- Cutler DM, Everett W. Thinking outside the pillbox—medication adherence as a priority for health care reform. N Engl J Med 2010;362(17):1553–5. http://dx.doi.org/10.1056/NEJMp1002305 - PubMed
-
- Hasford J, Uricher J, Tauscher M, Bramlage P, Virchow JC. Persistence with asthma treatment is low in Germany especially for controller medication: a population based study of 483,051 patients. Allergy 2010;65(3):347–54. http://dx.doi.org/10.1111/j.1398-9995.2009.02161.x - PubMed
-
- Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest 2000;117(2):542–50. http://dx.doi.org/10.1378/chest.117.2.542 - PubMed
-
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax 2008;63(9):831–8. http://dx.doi.org/10.1136/thx.2007.086041 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical