An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial
- PMID: 23460053
- PMCID: PMC3953144
- DOI: 10.7326/0003-4819-158-5-201303050-00002
An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial
Abstract
Background: Screening decreases colorectal cancer (CRC) incidence and mortality, yet almost half of age-eligible patients are not screened at recommended intervals.
Objective: To determine whether interventions using electronic health records (EHRs), automated mailings, and stepped increases in support improve CRC screening adherence over 2 years.
Design: 4-group, parallel-design, randomized, controlled comparative effectiveness trial with concealed allocation and blinded outcome assessments. (ClinicalTrials.gov: NCT00697047)
Setting: 21 primary care medical centers.
Patients: 4675 adults aged 50 to 73 years not current for CRC screening.
Intervention: Usual care, EHR-linked mailings ("automated"), automated plus telephone assistance ("assisted"), or automated and assisted plus nurse navigation to testing completion or refusal ("navigated"). Interventions were repeated in year 2.
Measurements: The proportion of participants current for screening in both years, defined as colonoscopy or sigmoidoscopy (year 1) or fecal occult blood testing (FOBT) in year 1 and FOBT, colonoscopy, or sigmoidoscopy (year 2).
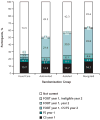
Results: Compared with those in the usual care group, participants in the intervention groups were more likely to be current for CRC screening for both years with significant increases by intensity (usual care, 26.3% [95% CI, 23.4% to 29.2%]; automated, 50.8% [CI, 47.3% to 54.4%]; assisted, 57.5% [CI, 54.5% to 60.6%]; and navigated, 64.7% [CI, 62.5% to 67.0%]; P < 0.001 for all pair-wise comparisons). Increases in screening were primarily due to increased uptake of FOBT being completed in both years (usual care, 3.9% [CI, 2.8% to 5.1%]; automated, 27.5% [CI, 24.9% to 30.0%]; assisted, 30.5% [CI, 27.9% to 33.2%]; and navigated, 35.8% [CI, 33.1% to 38.6%]).
Limitation: Participants were required to provide verbal consent and were more likely to be white and to participate in other types of cancer screening, limiting generalizability.
Conclusion: Compared with usual care, a centralized, EHR-linked, mailed CRC screening program led to twice as many persons being current for screening over 2 years. Assisted and navigated interventions led to smaller but significant stepped increases compared with the automated intervention only. The rapid growth of EHRs provides opportunities for spreading this model broadly.
Conflict of interest statement
Figures

Comment in
-
ACP Journal Club. Automated reminders and increasing intense support increased uptake of colorectal cancer screening.Ann Intern Med. 2013 Jun 18;158(12):JC8. doi: 10.7326/0003-4819-158-12-201306180-02008. Ann Intern Med. 2013. PMID: 23778932 No abstract available.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Vogelaar I, van Ballegooijen M, Schrag D, Boer R, Winawer SJ, Habbema JD, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–33. - PubMed
-
- Joseph DA, King JB, Miller JW, Richardson LC Centers for Disease Control and Prevention (CDC) Prevalence of colorectal cancer screening among adults—Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):51–6. - PubMed
-
- U.S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. - PubMed
-
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–58. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
