Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension
- PMID: 23460290
- PMCID: PMC3909776
- DOI: 10.1161/HYPERTENSIONAHA.111.00226
Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension
Abstract
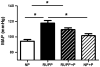
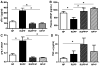
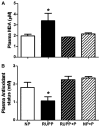
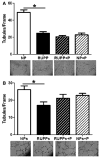
Preeclampsia is a pregnancy-specific condition characterized by an imbalance of circulating angiogenic factors and new-onset hypertension. Although current treatment options are limited, recent studies suggest that pravastatin may improve angiogenic profile and reduce blood pressure in preeclampsia. We hypothesized pravastatin would restore angiogenic balance and reduce mean arterial pressure (MAP) in rats with reduced utero-placental perfusion pressure (RUPP)-induced hypertension. Pravastatin was administered intraperitoneally (1 mg/kg per day) in RUPP (RUPP+P) and normal pregnant rats (NP+P) from day 14 to 19 of pregnancy. On day 19, MAP was measured via catheter, conceptus data were recorded, and tissues collected. MAP was increased (P<0.05) in RUPP compared with NP dams, and pravastatin ameliorated this difference. Pravastatin attenuated decreased fetal weight and plasma vascular endothelial growth factor and the RUPP-induced increased soluble fms-like tyrosine kinase-1 when compared with NP dams. Pravastatin treatment did not improve angiogenic potential in RUPP serum and decreased (P<0.05) endothelial tube formation in NP rats. RUPP rats presented with indices of oxidative stress, such as increased placental catalase activity and plasma thiobarbituric acid reactive substances along with decreased plasma total antioxidant capacity compared with NP controls, and pravastatin attenuated these effects. MAP, fetal weight, plasma vascular endothelial growth factor, and plasma soluble fms-like tyrosine kinase-1 were unchanged in NP+P compared with NP controls. The present data indicate that treatment with pravastatin attenuates oxidative stress and lowers MAP in placental ischemia-induced hypertension, but may have negative effects on circulating angiogenic potential during pregnancy. Further studies are needed to determine whether there are long-term deleterious effects on maternal or fetal health after pravastatin treatment during pregnancy-induced hypertension or preeclampsia.
Figures

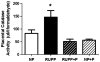

References
-
- August P, Lindheimer M. Pathophysiology of preeclampsia. In: Laragh J, Brenner BM, editors. Hypertension. New York: Raven Press; 1995. pp. 2407–2426.
-
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. - PubMed
-
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649–658. - PMC - PubMed
-
- Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. - PubMed
-
- Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

