The Plasmodium vivax merozoite surface protein 1 paralog is a novel erythrocyte-binding ligand of P. vivax
- PMID: 23460511
- PMCID: PMC3648005
- DOI: 10.1128/IAI.01117-12
The Plasmodium vivax merozoite surface protein 1 paralog is a novel erythrocyte-binding ligand of P. vivax
Abstract
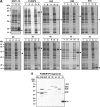
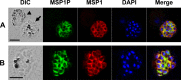
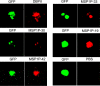
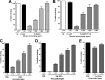
Merozoite surface protein 1 of Plasmodium vivax (PvMSP1), a glycosylphosphatidylinositol-anchored protein (GPI-AP), is a malaria vaccine candidate for P. vivax. The paralog of PvMSP1, named P. vivax merozoite surface protein 1 paralog (PvMSP1P; PlasmoDB PVX_099975), was recently identified and predicted as a GPI-AP. The similarities in genetic structural characteristics between PvMSP1 and PvMSP1P (e.g., size of open reading frames, two epidermal growth factor-like domains, and GPI anchor motif in the C terminus) led us to study this protein. In the present study, different regions of the PvMSP1P protein, demarcated based on the processed forms of PvMSP1, were expressed successfully as recombinant proteins [i.e., 83 (A, B, and C), 30, 38, 42, 33, and 19 fragments]. We studied the naturally acquired immune response against each fragment of recombinant PvMSP1P and the potential ability of each fragment to bind erythrocytes. The N-terminal fragment (83A) and two C-terminal fragments (33 and 19) reacted strongly with sera from P. vivax-infected patients, with 50 to 68% sensitivity and 95 to 96% specificity, respectively. Due to colocalization of PvMSP1P with PvMSP1, we supposed that PvMSP1P plays a similar role as PvMSP1 during erythrocyte invasion. An in vitro cytoadherence assay showed that PvMSP1P, especially the 19-kDa C-terminal region, could bind to erythrocytes. We also found that human sera from populations naturally exposed to vivax malaria and antisera obtained by immunization using the recombinant molecule PvMSP1P-19 inhibited in vitro binding of human erythrocytes to PvMSP1P-19. These results provide further evidence that the PvMSP1P might be an essential parasite adhesion molecule in the P. vivax merozoite and is a potential vaccine candidate against P. vivax.
Figures




References
-
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. 2009. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 9:555–566 - PubMed
-
- Gaur D, Mayer DC, Miller LH. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413–1429 - PubMed
-
- Cowman AF, Crabb BS. 2006. Invasion of red blood cells by malaria parasites. Cell 124:755–766 - PubMed
-
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. 1976. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 295:302–304 - PubMed
-
- Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, Bertrand O, Picot J, King CL, Grimberg BT, Mercereau-Puijalon O, Zimmerman PA. 2010. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc. Natl. Acad. Sci. U. S. A. 107:5967–5971 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

