Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway
- PMID: 23460563
- PMCID: PMC3606677
- DOI: 10.1213/ANE.0b013e3182860fc9
Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway
Abstract
Background: Growing evidence indicates that ketamine causes neurotoxicity in a variety of developing animal models, leading to a serious concern regarding the safety of pediatric anesthesia. However, if and how ketamine induces human neural cell toxicity is unknown. Recapitulation of neurogenesis from human embryonic stem cells (hESCs) in vitro allows investigation of the toxic effects of ketamine on neural stem cells (NSCs) and developing neurons, which is impossible to perform in humans. In the present study, we assessed the influence of ketamine on the hESC-derived NSCs and neurons.
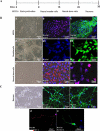
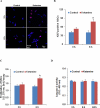
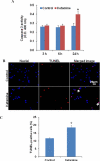
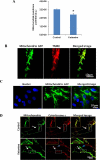
Methods: hESCs were directly differentiated into neurons via NSCs. NSCs and 2-week-old neurons were treated with varying doses of ketamine for different durations. NSC proliferation capacity was analyzed by Ki67 immunofluorescence staining and bromodeoxyuridine assay. Neuroapoptosis was analyzed by TUNEL staining and caspase 3 activity measurement. The mitochondria-related neuronal apoptosis pathway including mitochondrial membrane potential, cytochrome c distribution within cells, mitochondrial fission, and reactive oxygen species (ROS) production were also investigated.
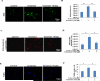
Results: Ketamine (100 µM) increased NSC proliferation after 6-hour exposure. However, significant neuronal apoptosis was only observed after 24 hours of ketamine treatment. In addition, ketamine decreased mitochondrial membrane potential and increased cytochrome c release from mitochondria into cytosol. Ketamine also enhanced mitochondrial fission as well as ROS production compared with no-treatment control. Importantly, Trolox, a ROS scavenger, significantly attenuated the increase of ketamine-induced ROS production and neuronal apoptosis.
Conclusions: These data for the first time demonstrate that (1) ketamine increases NSC proliferation and causes neuronal apoptosis; (2) mitochondria are involved in ketamine-induced neuronal toxicity, which can be prevented by Trolox; and (3) the stem cell-associated neurogenesis system may provide a simple and promising in vitro model for rapidly screening anesthetic neurotoxicity and studying the underlying mechanisms as well as prevention strategies to avoid this toxic effect.
Figures


References
-
- Chalon J, Tang CK, Ramanathan S, Eisner M, Katz R, Turndorf H. Exposure to halothane and enflurane affects learning function of murine progeny. Anesth Analg. 1981;60:794–7. - PubMed
-
- Mazoit JX, Roulleau P, Baujard C. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain: isoflurane or ischemia-reperfusion? Anesthesiology. 2010;113:1245. author reply -6. - PubMed
-
- Slikker W, Jr., Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicological sciences : an official journal of the Society of Toxicology. 2007;98:145–58. - PubMed
-
- Haley-Andrews S. Ketamine: the sedative of choice in a busy pediatric emergency department. J Emerg Nurs. 2006;32:186–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

