BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid
- PMID: 23460729
- PMCID: PMC3600908
- DOI: 10.1084/jem.20121088
BATF is required for normal expression of gut-homing receptors by T helper cells in response to retinoic acid
Abstract
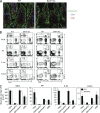
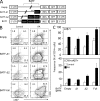
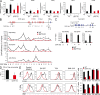
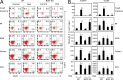
CCR9 and α4β7 are the major trafficking receptors for lymphocyte migration to the gut, and their expression is induced during lymphocyte activation under the influence of retinoic acid (RA). We report here that BATF (basic leucine zipper transcription factor, ATF-like), an AP-1 protein family factor, is required for optimal expression of CCR9 and α4β7 by T helper cells. BATF-deficient (knockout [KO]) mice had reduced numbers of effector T and regulatory T cells in the intestine. The intestinal T cells in BATF KO mice expressed CCR9 and α4β7 at abnormally low levels compared with their wild-type (WT) counterparts, and BATF KO CD4(+) T cells failed to up-regulate the expression of CCR9 and α4β7 to WT levels in response to RA. Defective binding of RARα and histone acetylation at the regulatory regions of the CCR9 and Itg-α4 genes were observed in BATF KO T cells. As a result, BATF KO effector and FoxP3(+) T cells failed to populate the intestine, and neither population functioned normally in the induction and regulation of colitis. Our results establish BATF as a cellular factor required for normal expression of CCR9 and α4β7 and for the homeostasis and effector functions of T cell populations in the intestine.
Figures





References
-
- Abitorabi M.A., Mackay C.R., Jerome E.H., Osorio O., Butcher E.C., Erle D.J. 1996. Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph. J. Immunol. 156:3111–3117 - PubMed
-
- Cassani B., Villablanca E.J., Quintana F.J., Love P.E., Lacy-Hulbert A., Blaner W.S., Sparwasser T., Snapper S.B., Weiner H.L., Mora J.R. 2011. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 141:2109–2118 10.1053/j.gastro.2011.09.015 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

