PRAME is a golgi-targeted protein that associates with the Elongin BC complex and is upregulated by interferon-gamma and bacterial PAMPs
- PMID: 23460923
- PMCID: PMC3584020
- DOI: 10.1371/journal.pone.0058052
PRAME is a golgi-targeted protein that associates with the Elongin BC complex and is upregulated by interferon-gamma and bacterial PAMPs
Erratum in
-
Correction: PRAME Is a Golgi-Targeted Protein That Associates with the Elongin BC Complex and Is Upregulated by Interferon-Gamma and Bacterial PAMPs.PLoS One. 2015 Jun 12;10(6):e0129297. doi: 10.1371/journal.pone.0129297. eCollection 2015. PLoS One. 2015. PMID: 26068868 Free PMC article. No abstract available.
Abstract
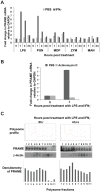
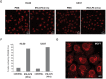
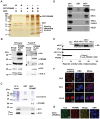
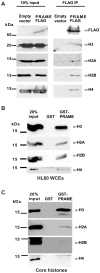
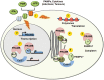
Preferentially expressed antigen in melanoma (PRAME) has been described as a cancer-testis antigen and is associated with leukaemias and solid tumours. Here we show that PRAME gene transcription in leukaemic cell lines is rapidly induced by exposure of cells to bacterial PAMPs (pathogen associated molecular patterns) in combination with type 2 interferon (IFNγ). Treatment of HL60 cells with lipopolysaccharide or peptidoglycan in combination with IFNγ resulted in a rapid and transient induction of PRAME transcription, and increased association of PRAME transcripts with polysomes. Moreover, treatment with PAMPs/IFNγ also modulated the subcellular localisation of PRAME proteins in HL60 and U937 cells, resulting in targeting of cytoplasmic PRAME to the Golgi. Affinity purification studies revealed that PRAME associates with Elongin B and Elongin C, components of Cullin E3 ubiquitin ligase complexes. This occurs via direct interaction of PRAME with Elongin C, and PRAME colocalises with Elongins in the Golgi after PAMP/IFNγ treatment. PRAME was also found to co-immunoprecipitate core histones, consistent with its partial localisation to the nucleus, and was found to bind directly to histone H3 in vitro. Thus, PRAME is upregulated by signalling pathways that are activated in response to infection/inflammation, and its product may have dual functions as a histone-binding protein, and in directing ubiquitylation of target proteins for processing in the Golgi.
Conflict of interest statement
Figures
References
-
- Ikeda H, Lethe B, Lehmann F, van Baren N, Baurain JF, et al. (1997) Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity 6: 199–208. - PubMed
-
- Atanackovic D, Luetkens T, Kloth B, Fuchs G, Cao Y, et al. (2011) Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol 86: 918–922. - PubMed
-
- Matsushita M, Ikeda H, Kizaki M, Okamoto S, Ogasawara M, et al. (2001) Quantitative monitoring of the PRAME gene for the detection of minimal residual disease in leukaemia. Br J Haematol 112: 916–926. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

