Connexin43 hemichannel-mediated regulation of connexin43
- PMID: 23460926
- PMCID: PMC3584027
- DOI: 10.1371/journal.pone.0058057
Connexin43 hemichannel-mediated regulation of connexin43
Abstract
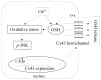
Background: Many signaling molecules and pathways that regulate gap junctions (GJs) protein expression and function are, in fact, also controlled by GJs. We, therefore, speculated an existence of the GJ channel-mediated self-regulation of GJs. Using a cell culture model in which nonjunctional connexin43 (Cx43) hemichannels were activated by cadmium (Cd(2+)), we tested this hypothesis.
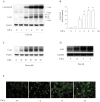
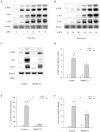
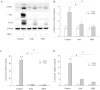
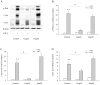
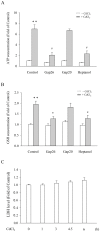
Principal findings: Incubation of Cx43-transfected LLC-PK1 cells with Cd(2+) led to an increased expression of Cx43. This effect of Cd(2+) was tightly associated with JNK activation. Inhibition of JNK abolished the elevation of Cx43. Further analysis revealed that the changes of JNK and Cx43 were controlled by GSH. Supplement of a membrane-permeable GSH analogue GSH ethyl ester or GSH precursor N-acetyl-cystein abrogated the effects of Cd(2+) on JNK activation and Cx43 expression. Indeed, Cd(2+) induced extracellular release of GSH. Blockade of Cx43 hemichannels with heptanol or Cx43 mimetic peptide Gap26 to prevent the efflux of GSH significantly attenuated the Cx43-elevating effects of Cd(2+).
Conclusions: Collectively, our results thus indicate that Cd(2+)-induced upregulation of Cx43 is through activation of nonjunctional Cx43 hemichannels. Our findings thus support the existence of a hemichannel-mediated self-regulation of Cx43 and provide novel insights into the molecular mechanisms of Cx43 expression and function.
Conflict of interest statement
Figures


References
-
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400. - PubMed
-
- Yao J, Zhu Y, Morioka T, Oite T, Kitamura M (2007) Pathophysiological roles of gap junction in glomerular mesangial cells. J Membr Biol 217: 123–130. - PubMed
-
- Yao J, Oite T, Kitamura M (2009) Gap junctional intercellular communication in the juxtaglomerular apparatus. Am J Physiol Renal Physiol 296: F939–946. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

