Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug
- PMID: 23461341
- PMCID: PMC3880422
- DOI: 10.1021/mp300595a
Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug
Abstract
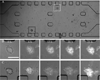
The importance and advantages of three-dimensional (3D) cell cultures have been well-recognized. Tumor cells cultured in a 3D culture system as multicellular tumor spheroids (MTS) can bridge the gap between in vitro and in vivo anticancer drug evaluations. An in vitro 3D tumor model capable of providing close predictions of in vivo drug efficacy will enhance our understanding, design, and development of better drug delivery systems. Here, we developed an in vitro 3D tumor model by adapting the hydrogel template strategy to culture uniformly sized spheroids in a hydrogel scaffold containing microwells. The in vitro 3D tumor model was to closely simulate an in vivo solid tumor and its microenvironment for evaluation of anticancer drug delivery systems. MTS cultured in the hydrogel scaffold are used to examine the effect of culture conditions on the drug responses. Free MTS released from the scaffold are transferred to a microfluidic channel to simulate a dynamic in vivo microenvironment. The in vitro 3D tumor model that mimics biologically relevant parameters of in vivo microenvironments such as cell-cell and cell-ECM interactions, and a dynamic environment would be a valuable device to examine efficiency of anticancer drug and targeting specificity. These models have potential to provide in vivo correlated information to improve and optimize drug delivery systems for an effective chemotherapy.
Figures






References
-
- Owen SC, Shoichet MS. Design of three-dimensional biomimetic scaffolds. J. Biomed. Mater. Res. Part A. 2010;94(4):1321–1331. - PubMed
-
- Abbott A. Cell culture: biology’s new dimension. Nature. 2003;424(6951):870–872. - PubMed
-
- Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Engineering tumors with 3D scaffolds. Nat. Methods. 2007;4(10):855–860. - PubMed
-
- Feder-Mengus C, Ghosh S, Reschner A, Martin I, Spagnoli GC. New dimensions in tumor immunology: what does 3D culture reveal? Trends Mol. Med. 2008;14(8):333–340. - PubMed
-
- Hamilton G. Multicellular spheroids as an in vitro tumor model. Cancer Lett. 1998;131(1):29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

