NHC-Cu-catalyzed protoboration of monosubstituted allenes. Ligand-controlled site selectivity, application to synthesis and mechanism
- PMID: 23461762
- PMCID: PMC3625862
- DOI: 10.1021/ol4004178
NHC-Cu-catalyzed protoboration of monosubstituted allenes. Ligand-controlled site selectivity, application to synthesis and mechanism
Abstract
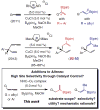
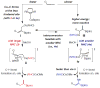
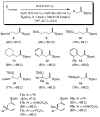
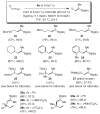
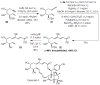
Two types of NHC-Cu complexes catalyze protoborations of terminal allenes to afford valuable 1,1- or trisubstituted vinylboron species with high site selectivity and stereoselectivity. The scope of the method, application to natural product synthesis, and mechanistic basis for the observed selectivity trends are presented.
Figures

References
-
-
For applications of vinylboron compounds in C–C bond formation, see: Hall DG. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley–VCH; Weimheim: 2005. . Tobisu M, Chatani N. Angew Chem Int Ed. 2009;48:3565.. For syntheses of cyclic and acyclic vinylborons by Pd-catalyzed cross-coupling reactions involving vinyl bromides and triflates, see: Takagi J, Takahashi K, Ishiyama T, Miyaura N. J Am Chem Soc. 2002;124:8001.. For a review on applications of vinyltrifluoroboron species, accessed via vinylborons, see: Molander GA, Ellis N. Acc Chem Res. 2007;40:275.
-
-
- Jang H, Zhugralin AR, Lee Y, Hoveyda AH. J Am Chem Soc. 2011;133:7859. - PubMed
-
-
For synthesis of vinylboron compounds by Cu-catalyzed protoboration of alkynes (in addition to ref 2), see: Kim HR, Jung IG, Yoo K, Jang K, Lee ES, Yun J, Son SU. Chem Commun. 2010;46:758.. Semba K, Fujihara T, Terao J, Tsuji Y. Chem Eur J. 2012;18:4179.. Moure AL, Arrayás RG, Gárdenas DJ, Alonso I, Carretero JC. J Am Chem Soc. 2012;134:7219.. Park JK, Ondrusek BA, McQuade DT. Org Lett. 2012;14:4790.
-
-
-
Subsequent to studies in ref. , a disclosure regarding site selective synthesis of vinylboron products by Cu-catalyzed protoboration of mono-substituted allenes appeared. However, catalysts were exclusively phosphine-based, substrates were almost all aryl-substituted, application to complex molecules synthesis was not demonstrated and detailed rationale for the origin of the observed selectivities was not provided. See: Yuan W, Ma S. Adv Synth Catal. 2012;354:1867.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

