Cancer stem-like cell properties are regulated by EGFR/AKT/β-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma
- PMID: 23461856
- PMCID: PMC3831031
- DOI: 10.1111/febs.12226
Cancer stem-like cell properties are regulated by EGFR/AKT/β-catenin signaling and preferentially inhibited by gefitinib in nasopharyngeal carcinoma
Abstract
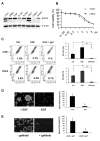
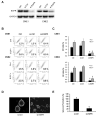
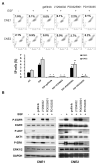
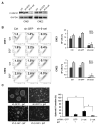
We report that the epidermal growth factor receptor (EGFR) pathway plays a critical role in regulating cancer stem-like cells (CSCs) in nasopharyngeal carcinoma (NPC), one of the most common malignant tumors in Southeast Asia. Effects of EGFR on maintaining CSCs are mainly mediated by AKT signaling, and β-catenin is responsible for governing CSC properties in response to EGFR/AKT activation. Significantly, CSCs are enriched by cisplatin and decreased by gefitinib in NPC xenograft models. Upon reimplantation in secondary mice, tumor cells derived from cisplatin-treated mice grew rapidly, whereas regrowth of tumor cells from gefitinib-treated mice was severely diminished. We further demonstrate that expression of EGFR correlates with expression of β-catenin and Nanog in primary tumor specimens from NPC patients. These findings provide mechanistic and preclinical evidence supporting the use of gefitinib alone or in combination with a chemotherapeutic agent in first-line therapy for patients with NPC. In addition, our results suggest that targeting β-catenin represents a rational clinical modality for patients whose tumors harbor activated EGFR or AKT.
© 2013 The Authors Journal compilation © 2013 FEBS.
Figures

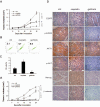
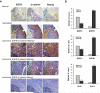
References
-
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene (Amsterdam) 2006;366:2–16. - PubMed
-
- Eccles SA. The epidermal growth factor receptor/Erb-B/HER family in normal and malignant breast biology. Int J Dev Biol. 2011;55:685–96. - PubMed
-
- Reynolds BA, Weiss S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central-Nervous-System. Science. 1992;255:1707–1710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

