Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: the MsFLASH experience
- PMID: 23462342
- PMCID: PMC3670607
- DOI: 10.1016/j.cct.2013.02.009
Design and methods of a multi-site, multi-behavioral treatment trial for menopausal symptoms: the MsFLASH experience
Abstract
Background: Behavioral strategies are recommended for menopausal symptoms, but little evidence exists regarding efficacy.
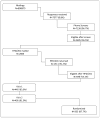
Purpose: Describe design and methodology of a randomized controlled 3 by 2 factorial trial of yoga, exercise and omega-3 fatty acids.
Methods: Women from three geographic areas with a weekly average of ≥14 hot flashes/night sweats, who met exclusion/inclusion criteria, were randomized to 12weeks of: 1) yoga classes and daily home practice; 2) supervised, facility-based aerobic exercise training; or 3) usual activity. Women in each arm were further randomized to either omega-3 supplement or placebo. Standardized training, on-going monitoring, and site visits were adopted to ensure consistency across sites and fidelity to the intervention. Participant adherence to the intervention protocol was monitored continuously, and retention was actively encouraged by staff. Information on adverse events was systematically collected.
Results: Of 7377 women who responded to mass mailings, 355 (4.8%) were randomized; mean age was 54.7 (sd=3.7), 26.2% were African American, 81.7% were post-menopausal, and mean baseline frequency of daily hot flashes/night sweats was 7.6 (sd=3.8). Adherence of ≥80% was 59% for yoga, 77% for exercise training, and 80% for study pills. Final week 12 data were collected from 95.2%
Conclusions: Conducting a multi-site, multi-behavioral randomized trial for menopausal symptoms is challenging but feasible. Benefits included cost-effective study design, centralized recruitment, and methodologic standardization.
Trial registration: ClinicalTrials.gov NCT01178892.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43. - PubMed
-
- Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62:153–159. - PubMed
-
- Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–358. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01AG032659/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01AG032699/AG/NIA NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01AG032700/AG/NIA NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01AG032669/AG/NIA NIH HHS/United States
- U01 AG032656/AG/NIA NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- U01AG032682/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous