Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis
- PMID: 23463691
- PMCID: PMC3888615
- DOI: 10.1136/annrheumdis-2012-202726
Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis
Abstract
Background: Rheumatoid arthritis (RA) is characterised by autoimmunity to citrullinated proteins, and there is increasing epidemiologic evidence linking Porphyromonas gingivalis to RA. P gingivalis is apparently unique among periodontal pathogens in possessing a citrullinating enzyme, peptidylarginine deiminase (PPAD) with the potential to generate antigens driving the autoimmune response.
Objectives: To examine the immune response to PPAD in patients with RA, individuals with periodontitis (PD) and controls (without arthritis), confirm PPAD autocitrullination and identify the modified arginine residues.
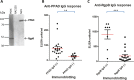
Methods: PPAD and an inactivated mutant (C351A) were cloned and expressed and autocitrullination of both examined by immunoblotting and mass spectrometry. ELISAs using PPAD, C351A and another P gingivalis protein arginine gingipain (RgpB) were developed and antibody reactivities examined in patients with RA (n=80), individuals with PD (n=44) and controls (n=82).
Results: Recombinant PPAD was a potent citrullinating enzyme. Antibodies to PPAD, but not to Rgp, were elevated in the RA sera (median 122 U/ml) compared with controls (median 70 U/ml; p<0.05) and PD (median 60 U/ml; p<0.01). Specificity of the anti-peptidyl citrullinated PPAD response was confirmed by the reaction of RA sera with multiple epitopes tested with synthetic citrullinated peptides spanning the PPAD molecule. The elevated antibody response to PPAD was abolished in RA sera if the C351A mutant was used on ELISA.
Conclusions: The peptidyl citrulline-specific immune response to PPAD supports the hypothesis that, as a bacterial protein, it might break tolerance in RA, and could be a target for therapy.
Keywords: Ant-CCP; Autoimmunity; Rheumatoid Arthritis.
Figures




References
-
- Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev 2010;233:34–54 - PubMed
-
- Vossenaar ER, Zendman AJ, van Venrooij WJ, et al. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003;25:1106–18 - PubMed
-
- Vossenaar ER, Smeets TJ, Kraan MC, et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum 2004;50:3485–94 - PubMed
-
- Foulquier C, Sebbag M, Clavel C, et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum 2007;56:3541–53 - PubMed
-
- Kinloch A, Lundberg K, Wait R, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum 2008;58:2287–95 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

