Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy
- PMID: 23464801
- PMCID: PMC3618492
- DOI: 10.1111/epi.12118
Ethosuximide reduces epileptogenesis and behavioral comorbidity in the GAERS model of genetic generalized epilepsy
Abstract
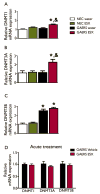
Purpose: Ethosuximide (ESX) is a drug of choice for the symptomatic treatment of absence seizures. Chronic treatment with ESX has been reported to have disease-modifying antiepileptogenic activity in the WAG/Rij rat model of genetic generalized epilepsy (GGE) with absence seizures. Here we examined whether chronic treatment with ESX (1) possesses antiepileptogenic effects in the genetic absence epilepsy rats from Strasbourg (GAERS) model of GGE, (2) is associated with a mitigation of behavioral comorbidities, and (3) influences gene expression in the somatosensory cortex region where seizures are thought to originate.
Methods: GAERS and nonepileptic control (NEC) rats were chronically treated with ESX (in drinking water) or control (tap water) from 3 to 22 weeks of age. Subsequently, all animals received tap water only for another 12 weeks to assess enduring effects of treatment. Seizure frequency and anxiety-like behaviors were serially assessed throughout the experimental paradigm. Treatment effects on the expression of key components of the epigenetic molecular machinery, the DNA methyltransferase enzymes, were assessed using quantitative polymerase chain reaction (qPCR).
Key findings: ESX treatment significantly reduced seizures in GAERS during the treatment phase, and this effect was maintained during the 12-week posttreatment phase (p < 0.05). Furthermore, the anxiety-like behaviors present in GAERS were reduced by ESX treatment (p < 0.05). Molecular analysis revealed that ESX treatment was associated with increased expression of DNA methyltransferase enzyme messenger RNA (mRNA) in cortex.
Significance: Chronic ESX treatment has disease-modifying effects in the GAERS model of GGE, with antiepileptogenic effects against absence seizures and mitigation of behavioral comorbidities. The cellular mechanism for these effects may involve epigenetic modifications.
Wiley Periodicals, Inc. © 2013 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest, financial or otherwise, to disclose.
Figures



References
-
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. - PMC - PubMed
-
- Bouilleret V, Hogan RE, Velakoulis D, Salzberg MR, Wang L, Egan GF, O’Brien TJ, Jones NC. Morphometric abnormalities and hyperanxiety in genetically epileptic rats: a model of psychiatric comorbidity? Neuroimage. 2009;45:267–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

