Renal and extrarenal actions of Klotho
- PMID: 23465499
- PMCID: PMC3593734
- DOI: 10.1016/j.semnephrol.2012.12.013
Renal and extrarenal actions of Klotho
Abstract
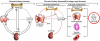
Klotho is a single-pass transmembrane protein highly expressed in the kidney. Membrane Klotho protein acts as a co-receptor for fibroblast growth factor-23. Its extracellular domain is shed from the cell surface and functions as an endocrine substance that exerts multiple renal and extrarenal functions. An exhaustive review is beyond the scope and length of this article; thus, only effects with pertinence to mineral metabolism and renoprotection are highlighted here. Klotho participates in mineral homeostasis via interplay with other calciophosphoregulatory hormones (parathyroid hormone, fibroblast growth factor-23, and 1,25-[OH]2 vitamin D3) in kidney, bone, intestine, and parathyroid gland. Klotho also may be involved in acute and chronic kidney disease development and progression. Acute kidney injury is a temporary and reversible state of Klotho deficiency and chronic kidney disease is a sustained state of systemic Klotho deficiency. Klotho deficiency renders the kidney more susceptible to acute insults, delays kidney regeneration, and promotes renal fibrosis. In addition to direct renal effects, Klotho deficiency also triggers and aggravates deranged mineral metabolism, secondary hyperparathyroidism, vascular calcification, and cardiac hypertrophy and fibrosis. Although studies examining the therapeutic effect of Klotho replacement were performed in animal models, it is quite conceivable that supplementation of exogenous Klotho and/or up-regulation of endogenous Klotho production may be a viable therapeutic strategy for patients with acute or chronic kidney diseases.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. - PubMed
-
- Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. 2000;267:597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

