A zebrafish model of conditional targeted podocyte ablation and regeneration
- PMID: 23466998
- PMCID: PMC3672345
- DOI: 10.1038/ki.2013.6
A zebrafish model of conditional targeted podocyte ablation and regeneration
Abstract
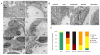
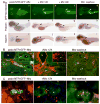
Podocytes are specialized cells that contribute critically to the normal structure and function of the glomerular filtration barrier. Their depletion plays an important role in the pathogenesis of glomerulosclerosis. Here, we report generation of a genetic model of conditional podocyte ablation and regeneration in zebrafish using a bacterial nitroreductase strategy to convert a prodrug, metronidazole, into a cytotoxic metabolite. A transgenic zebrafish line was generated that expresses green fluorescence protein (GFP) and the nitroreductase fusion protein under the control of the podocin promoter Tg(podocin:nitroreductase-GFP). Treatment of these transgenic zebrafish with metronidazole results in podocyte apoptosis, a loss of nephrin and podocin expression, foot process effacement, and a leaky glomerular filtration barrier. Following metronidazole washout, proliferating cells were detected in the glomeruli of recovering transgenic fish with a restoration of nitroreductase-GFP fluorescence, nephrin and podocin expression, a reestablishment of normal foot process architecture, and glomerular barrier function. Thus, our studies show that zebrafish podocytes are capable of regenerating following depletion, and establish the Tg(podocin:NTR-GFP) fish as a new model to study podocyte injury and repair.
Conflict of interest statement
None
Figures


References
-
- Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. - PubMed
-
- Moreno JA, Sanchez-Nino MD, Sanz AB, et al. A slit in podocyte death. Curr Med Chem. 2008;15:1645–1654. - PubMed
-
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
-
- Hishiki T, Shirato I, Takahashi Y, et al. Podocyte injury predicts prognosis in patients with iga nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press Res. 2001;24:99–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

