Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells
- PMID: 23467095
- PMCID: PMC3746493
- DOI: 10.1038/nature11868
Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells
Abstract
There has been a marked increase in the incidence of autoimmune diseases in the past half-century. Although the underlying genetic basis of this class of diseases has recently been elucidated, implicating predominantly immune-response genes, changes in environmental factors must ultimately be driving this increase. The newly identified population of interleukin (IL)-17-producing CD4(+) helper T cells (TH17 cells) has a pivotal role in autoimmune diseases. Pathogenic IL-23-dependent TH17 cells have been shown to be critical for the development of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis, and genetic risk factors associated with multiple sclerosis are related to the IL-23-TH17 pathway. However, little is known about the environmental factors that directly influence TH17 cells. Here we show that increased salt (sodium chloride, NaCl) concentrations found locally under physiological conditions in vivo markedly boost the induction of murine and human TH17 cells. High-salt conditions activate the p38/MAPK pathway involving nuclear factor of activated T cells 5 (NFAT5; also called TONEBP) and serum/glucocorticoid-regulated kinase 1 (SGK1) during cytokine-induced TH17 polarization. Gene silencing or chemical inhibition of p38/MAPK, NFAT5 or SGK1 abrogates the high-salt-induced TH17 cell development. The TH17 cells generated under high-salt conditions display a highly pathogenic and stable phenotype characterized by the upregulation of the pro-inflammatory cytokines GM-CSF, TNF-α and IL-2. Moreover, mice fed with a high-salt diet develop a more severe form of EAE, in line with augmented central nervous system infiltrating and peripherally induced antigen-specific TH17 cells. Thus, increased dietary salt intake might represent an environmental risk factor for the development of autoimmune diseases through the induction of pathogenic TH17 cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
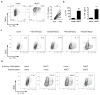
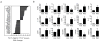
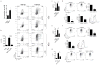
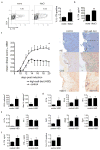
Comment in
-
Autoimmunity: Rubbing salt in the wound.Nature. 2013 Apr 25;496(7446):437-9. doi: 10.1038/nature11959. Epub 2013 Mar 6. Nature. 2013. PMID: 23467087 No abstract available.
-
T cells: Salt promotes pathogenic TH17 cells.Nat Rev Immunol. 2013 Apr;13(4):225. doi: 10.1038/nri3432. Epub 2013 Mar 15. Nat Rev Immunol. 2013. PMID: 23493117 No abstract available.
-
PD research round-up: Sodium sensing: link to (auto)immunity.Perit Dial Int. 2013 Jul-Aug;33(4):348. doi: 10.3747/pdi.2013.00143. Perit Dial Int. 2013. PMID: 23843585 Free PMC article. No abstract available.
-
High sodium chloride consumption enhances the effects of smoking but does not interact with SGK1 polymorphisms in the development of ACPA-positive status in patients with RA.Ann Rheum Dis. 2016 May;75(5):943-6. doi: 10.1136/annrheumdis-2015-209009. Epub 2016 Feb 22. Ann Rheum Dis. 2016. PMID: 26903441 No abstract available.
References
-
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. - PubMed
-
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007;61:504–13. - PubMed
-
- Appel LJ, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

