HIV compromises integrity of the podocyte actin cytoskeleton through downregulation of the vitamin D receptor
- PMID: 23467424
- PMCID: PMC3680691
- DOI: 10.1152/ajprenal.00717.2012
HIV compromises integrity of the podocyte actin cytoskeleton through downregulation of the vitamin D receptor
Abstract
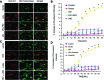
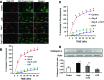
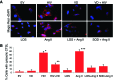
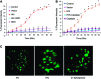
Alterations in the podocyte actin cytoskeleton have been implicated in the development of proteinuric kidney diseases. In the present study, we evaluated the effect of HIV on the podocyte actin cytoskeleton and the mechanism involved. We hypothesized that HIV may be compromising the actin cytoskeleton via downregulation of the vitamin D receptor (VDR) of conditionally immortalized differentiated human podocytes (CIDHPs). HIV-transduced podocytes (HIV/CIDHPs) not only displayed downregulation of VDR but also showed activation of the renin-angiotensin system (RAS) in the form of enhanced expression of renin and increased production of ANG II. Moreover, CIDHPs lacking VDR displayed enhanced ANG II production, and treatment of HIV/CIDHPs with EB1089 (vitamin D3; VD) attenuated ANG II production. HIV/CIDHPs as well as ANG II-treated CIDHPs exhibited enhanced expression of cathepsin (CTS) L. Additionally, losartan (an ANG II type I receptor blocker) inhibited both HIV- and ANG II-induced podocyte cathepsin L expression. Furthermore, VD downregulated HIV-induced podocyte CTSL expression. Both losartan and free radical scavengers attenuated HIV- and ANG II-induced podocyte reactive oxygen species (ROS) generation. HIV also led to cytosolic CTSL accumulation through enhancement of podocyte lysosomal membrane permeabilization; on the other hand, VD, losartan, and superoxide dismutase (SOD) attenuated HIV-induced enhanced podocyte cytosolic CTSL accumulation. Morphological evaluation of HIV/CIDHPs revealed sparse actin filaments and attenuated expression of dynamin. Interestingly, podocytes lacking CTSL displayed enhanced dynamin expression, and HIV/CIDHPs expressing CTSL exhibited downregulation of dynamin. These findings indicate that HIV-induced downregulation of podocyte VDR and associated RAS activation and cytosolic CTSL accumulation compromised the actin cytoskeleton.
Keywords: HIV-associated nephropathy; cathepsin L; podocyte; renin-angiotensin system; vitamin D receptor.
Figures




Similar articles
-
HIV-induced kidney cell injury: role of ROS-induced downregulated vitamin D receptor.Am J Physiol Renal Physiol. 2012 Aug 15;303(4):F503-14. doi: 10.1152/ajprenal.00170.2012. Epub 2012 May 30. Am J Physiol Renal Physiol. 2012. PMID: 22647636 Free PMC article.
-
Hyperglycemia enhances kidney cell injury in HIVAN through down-regulation of vitamin D receptors.Cell Signal. 2015 Mar;27(3):460-9. doi: 10.1016/j.cellsig.2014.12.011. Epub 2014 Dec 24. Cell Signal. 2015. PMID: 25542307 Free PMC article.
-
Angiotensin II-mediated MYH9 downregulation causes structural and functional podocyte injury in diabetic kidney disease.Sci Rep. 2019 May 22;9(1):7679. doi: 10.1038/s41598-019-44194-3. Sci Rep. 2019. PMID: 31118506 Free PMC article.
-
Podocytopathy: The role of actin cytoskeleton.Biomed Pharmacother. 2022 Dec;156:113920. doi: 10.1016/j.biopha.2022.113920. Epub 2022 Oct 27. Biomed Pharmacother. 2022. PMID: 36411613 Review.
-
Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review.
Cited by
-
Impact of vitamin D on immune function: lessons learned from genome-wide analysis.Front Physiol. 2014 Apr 21;5:151. doi: 10.3389/fphys.2014.00151. eCollection 2014. Front Physiol. 2014. PMID: 24795646 Free PMC article. Review.
-
HIV therapy, metabolic and cardiovascular health are associated with glomerular hyperfiltration among men with and without HIV infection.AIDS. 2014 Jan 28;28(3):377-86. doi: 10.1097/QAD.0000000000000094. AIDS. 2014. PMID: 24670523 Free PMC article.
-
Molecular Mechanisms of Proteinuria in Focal Segmental Glomerulosclerosis.Front Med (Lausanne). 2018 Apr 16;5:98. doi: 10.3389/fmed.2018.00098. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29713631 Free PMC article. Review.
-
Implications of Oxidative Stress and Potential Role of Mitochondrial Dysfunction in COVID-19: Therapeutic Effects of Vitamin D.Antioxidants (Basel). 2020 Sep 21;9(9):897. doi: 10.3390/antiox9090897. Antioxidants (Basel). 2020. PMID: 32967329 Free PMC article. Review.
-
Vitamin D receptor and epigenetics in HIV infection and drug abuse.Front Microbiol. 2015 Aug 19;6:788. doi: 10.3389/fmicb.2015.00788. eCollection 2015. Front Microbiol. 2015. PMID: 26347716 Free PMC article. Review.
References
-
- Atta MG, Lucas GM, Fine DM. HIV-associated nephropathy: epidemiology, pathogenesis, diagnosis and management. Expert Rev Anti Infect Ther 6: 365–371, 2008 - PubMed
-
- Barisoni L, Kriz W, Mundel P, D'agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 - PubMed
-
- Becker BN, Yasuda T, Kondo S, Vaikunth S, Homma T, Harris RC. Mechanical stretch/relaxation stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Exp Nephrol 6: 57–66, 1998 - PubMed
-
- Bird JE, Durham SK, Giancarli Gitliz PH, Pandya DG, Dambach DM, Mozes MM, Kopp JB. Captopril prevents nephropathy in HIV-transgenic mice. J Am Soc Nephrol 9: 1441–1447, 1998 - PubMed
-
- Burns GC, Paul SK, Toth IR, Sivak SL. Effect of angiotensin-converting enzyme inhibition in HIV-associated nephropathy. J Am Soc Nephrol 8: 1140–1146, 1997 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

