Arkadia regulates tumor metastasis by modulation of the TGF-β pathway
- PMID: 23467611
- PMCID: PMC3672972
- DOI: 10.1158/0008-5472.CAN-12-1916
Arkadia regulates tumor metastasis by modulation of the TGF-β pathway
Abstract
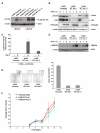
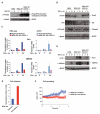
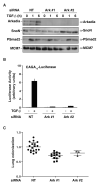
TGF-β can act as a tumor suppressor at early stages of cancer progression and as a tumor promoter at later stages. The E3 ubiquitin ligase Arkadia (RNF111) is a critical component of the TGF-β signaling pathway, being required for a subset of responses, those mediated by Smad3-Smad4 complexes. It acts by mediating ligand-induced degradation of Ski and SnoN (SKIL), which are 2 potent transcriptional repressors. Here, we investigate the role of Arkadia in cancer using model systems to address both potential tumor-suppressive and tumor-promoting roles. Stable reexpression of Arkadia in lung carcinoma NCI-H460 cells, which we show contain a hemizygous nonsense mutation in the Arkadia/RNF111 gene, efficiently restored TGF-β-induced Smad3-dependent transcription, and substantially decreased the ability of these cells to grow in soft agar in vitro. However, it had no effect on tumor growth in vivo in mouse models. Moreover, loss of Arkadia in cancer cell lines and human tumors is rare, arguing against a prominent tumor-suppressive role. In contrast, we have uncovered a potent tumor-promoting function for Arkadia. Using 3 different cancer cell lines whose tumorigenic properties are driven by TGF-β signaling, we show that loss of Arkadia function, either by overexpression of dominant negative Arkadia or by siRNA-induced knockdown, substantially inhibited lung colonization in tail vein injection experiments in immunodeficient mice. Our findings indicate that Arkadia is not critical for regulating tumor growth per se, but is required for the early stages of cancer cell colonization at the sites of metastasis.
Figures



References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Padua D, Massague J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

