Dual-color-emitting green fluorescent protein from the sea cactus Cavernularia obesa and its use as a pH indicator for fluorescence microscopy
- PMID: 23468077
- PMCID: PMC3884763
- DOI: 10.1002/bio.2497
Dual-color-emitting green fluorescent protein from the sea cactus Cavernularia obesa and its use as a pH indicator for fluorescence microscopy
Abstract
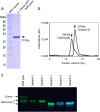
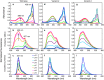
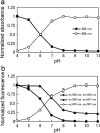
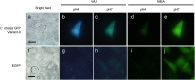
We isolated and characterized a green fluorescent protein (GFP) from the sea cactus Cavernularia obesa. This GFP exists as a dimer and has absorption maxima at 388 and 498 nm. Excitation at 388 nm leads to blue fluorescence (456 nm maximum) at pH 5 and below, and green fluorescence (507 nm maximum) at pH 7 and above, and the GFP is remarkably stable at pH 4. Excitation at 498 nm leads to green fluorescence (507 nm maximum) from pH 5 to pH 9. We introduced five amino acid substitutions so that this GFP formed monomers rather than dimers and then used this monomeric form to visualize intracellular pH change during the phagocytosis of living cells by use of fluorescence microscopy. The intracellular pH change is visualized by use of a simple long-pass emission filter with single-wavelength excitation, which is technically easier to use than dual-emission fluorescent proteins that require dual-wavelength excitation.
Keywords: GFP, dual-color emission; fluorescence microscopy; pH indicator; sea cactus.
Copyright © 2013 John Wiley & Sons, Ltd.
Figures




References
-
- Haddock SHD, Moline MA, Case JF. Bioluminescence in the sea. Annu Rev Mar Sci. 2010;2:443–93. - PubMed
-
- Shimomura O. Bioluminescence: chemical principles and methods. Singapore: World Scientific Publishing; 2006.
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–44. - PubMed
-
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–7. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

