Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications
- PMID: 23468415
- PMCID: PMC3663257
- DOI: 10.1007/s10928-013-9306-4
Cerebral microdialysis in clinical studies of drugs: pharmacokinetic applications
Abstract
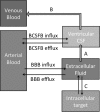
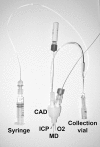
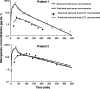
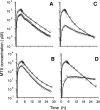
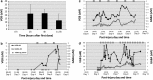
The ability to deliver drug molecules effectively across the blood-brain barrier into the brain is important in the development of central nervous system (CNS) therapies. Cerebral microdialysis is the only existing technique for sampling molecules from the brain extracellular fluid (ECF; also termed interstitial fluid), the compartment to which the astrocytes and neurones are directly exposed. Plasma levels of drugs are often poor predictors of CNS activity. While cerebrospinal fluid (CSF) levels of drugs are often used as evidence of delivery of drug to brain, the CSF is a different compartment to the ECF. The continuous nature of microdialysis sampling of the ECF is ideal for pharmacokinetic (PK) studies, and can give valuable PK information of variations with time in drug concentrations of brain ECF versus plasma. The microdialysis technique needs careful calibration for relative recovery (extraction efficiency) of the drug if absolute quantification is required. Besides the drug, other molecules can be analysed in the microdialysates for information on downstream targets and/or energy metabolism in the brain. Cerebral microdialysis is an invasive technique, so is only useable in patients requiring neurocritical care, neurosurgery or brain biopsy. Application of results to wider patient populations, and to those with different pathologies or degrees of pathology, obviously demands caution. Nevertheless, microdialysis data can provide valuable guidelines for designing CNS therapies, and play an important role in small phase II clinical trials. In this review, we focus on the role of cerebral microdialysis in recent clinical studies of antimicrobial agents, drugs for tumour therapy, neuroprotective agents and anticonvulsants.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

