Effects of postchallenge administration of ST-246 on dissemination of IHD-J-Luc vaccinia virus in normal mice and in immune-deficient mice reconstituted with T cells
- PMID: 23468500
- PMCID: PMC3648177
- DOI: 10.1128/JVI.03426-12
Effects of postchallenge administration of ST-246 on dissemination of IHD-J-Luc vaccinia virus in normal mice and in immune-deficient mice reconstituted with T cells
Abstract
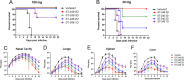
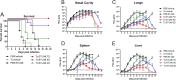
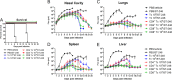
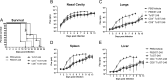
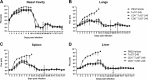
Whole-body bioimaging was used to study dissemination of vaccinia virus (VACV) in normal and in immune deficient (nu(-)/nu(-)) mice protected from lethality by postchallenge administration of ST-246. Total fluxes were recorded in the liver, spleen, lungs, and nasal cavities of live mice after intranasal infection with a recombinant IHD-J-Luc VACV expressing luciferase. Areas under the flux curve were calculated for individual mice to assess viral loads. Treatment for 2 to 5 days of normal BALB/c mice with ST-246 at 100 mg/kg starting 24 h postchallenge conferred 100% protection and reduced viral loads in four organs compared to control mice. Mice also survived after 5 days of treatment with ST-246 at 30 mg/kg, and yet the viral loads and poxes were higher in these mice compared to 100-mg/kg treatment group. Nude mice were not protected by ST-246 alone or by 10 million adoptively transferred T cells. In contrast, nude mice that received T cells and 7-day treatment with ST-246 survived infection and exhibited reduced viral loads compared to nonreconstituted and ST-246-treated mice after ST-246 was stopped. Similar protection of nude mice was achieved using adoptively transferred 1.0 and 0.1 million, but not 0.01 million, purified T cells or CD4(+) or CD8(+) T cells in conjunction with ST-246 treatment. These data suggest that ST-246 protects immunocompetent mice from lethality and reduces viral dissemination in internal organs and poxvirus lesions. Furthermore, immune-deficient animals with partial T cell reconstitution can control virus replication after a course of ST-246 and survive lethal vaccinia virus challenge.
Figures



References
-
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling PB, Hauer J, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl T, Russell PK, Tonat K. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127–2137 - PubMed
-
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, Wolfe ND, Shongo RL, Graham BS, Formenty P, Okitolonda E, Hensley LE, Meyer H, Wright LL, Muyembe JJ. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 107:16262–16267 - PMC - PubMed
-
- Whitley RJ. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 57:7–12 - PubMed
-
- Amanna IJ, Slifka MK, Crotty S. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211:320–337 - PubMed
-
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

