Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules
- PMID: 23468504
- PMCID: PMC3648192
- DOI: 10.1128/JVI.03134-12
Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules
Abstract
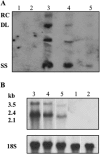
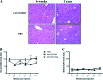
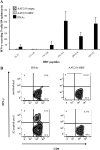
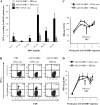
Hepatitis B virus (HBV) persistence may be due to impaired HBV-specific immune responses being unable to eliminate efficiently or cure infected hepatocytes. The immune mechanisms that lead to HBV persistence have not been completely identified, and no appropriate animal model is available for such studies. Therefore, we established a chronic HBV infection model in a mouse strain with human leukocyte antigen A2/DR1 (HLA-A2/DR1) transgenes and an H-2 class I/class II knockout. The liver of these mice was transduced with adeno-associated virus serotype 2/8 (AAV2/8) carrying a replication-competent HBV DNA genome. In all AAV2/8-transduced mice, hepatitis B virus surface antigen, hepatitis B virus e antigen, and HBV DNA persisted in serum for at least 1 year. Viral replication intermediates and transcripts were detected in the livers of the AAV-injected mice. The hepatitis B core antigen was expressed in 60% of hepatocytes. No significant inflammation was observed in the liver. This was linked to a higher number of regulatory T cells in liver than in controls and a defect in HBV-specific functional T-cell responses. Despite the substantial tolerance resulting from expression of HBV antigens in hepatocytes, we succeeded in priming functional HBV-specific T-cell responses in peripheral tissues, which subsequently reached the liver. This AAV2/8-HBV-transduced HLA-A2/DR1 murine model recapitulates virological and immunological characteristics of chronic HBV infection, and it could be useful for the development of new treatments and immune-based therapies or therapeutic vaccines for chronic HBV infections.
Figures


References
-
- Ganem D, Prince AM. 2004. Hepatitis B virus infection—natural history and clinical consequences. N. Engl. J. Med. 350:1118–1129 - PubMed
-
- Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 - PubMed
-
- Maini MK, Schurich A. 2010. The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J. Hepatol. 52:616–619 - PubMed
-
- Chisari FV. 1996. Hepatitis B virus transgenic mice: models of viral immunobiology and pathogenesis. Curr. Top. Microbiol. Immunol. 206:149–173 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

