Intramolecular interactions control Vms1 translocation to damaged mitochondria
- PMID: 23468520
- PMCID: PMC3639040
- DOI: 10.1091/mbc.E13-02-0072
Intramolecular interactions control Vms1 translocation to damaged mitochondria
Abstract
Mitochondrial dysfunction is associated with the development of many age-related human diseases. Therefore recognizing and correcting the early signs of malfunctioning mitochondria is of critical importance for cellular welfare and survival. We previously demonstrated that VCP/Cdc48-associated mitochondrial stress responsive 1 (Vms1) is a component of a mitochondrial surveillance system that mediates the stress-responsive degradation of mitochondrial proteins by the proteasome. Here we propose novel mechanisms through which Vms1 monitors the status of mitochondria and is recruited to damaged or stressed mitochondria. We find that Vms1 contains a highly conserved region that is necessary and sufficient for mitochondrial targeting (the mitochondrial targeting domain [MTD]). Of interest, MTD-mediated mitochondrial targeting of Vms1 is negatively regulated by a direct interaction with the Vms1 N-terminus. Using laser-induced generation of mitochondrial reactive oxygen species, we also show that Vms1 is preferentially recruited to mitochondria subjected to oxidative stress. These studies define cellular and biochemical mechanisms by which Vms1 locali-zation to mitochondria is controlled to enable an efficient protein quality control system.
Figures
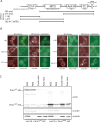



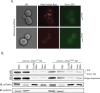
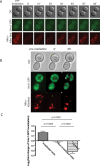
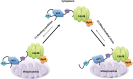
References
-
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

