Viral escape from neutralizing antibodies in early subtype A HIV-1 infection drives an increase in autologous neutralization breadth
- PMID: 23468623
- PMCID: PMC3585129
- DOI: 10.1371/journal.ppat.1003173
Viral escape from neutralizing antibodies in early subtype A HIV-1 infection drives an increase in autologous neutralization breadth
Abstract
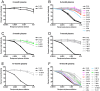
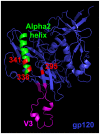
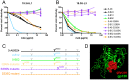
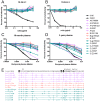
Antibodies that neutralize (nAbs) genetically diverse HIV-1 strains have been recovered from a subset of HIV-1 infected subjects during chronic infection. Exact mechanisms that expand the otherwise narrow neutralization capacity observed during early infection are, however, currently undefined. Here we characterized the earliest nAb responses in a subtype A HIV-1 infected Rwandan seroconverter who later developed moderate cross-clade nAb breadth, using (i) envelope (Env) glycoproteins from the transmitted/founder virus and twenty longitudinal nAb escape variants, (ii) longitudinal autologous plasma, and (iii) autologous monoclonal antibodies (mAbs). Initially, nAbs targeted a single region of gp120, which flanked the V3 domain and involved the alpha2 helix. A single amino acid change at one of three positions in this region conferred early escape. One immunoglobulin heavy chain and two light chains recovered from autologous B cells comprised two mAbs, 19.3H-L1 and 19.3H-L3, which neutralized the founder Env along with one or three of the early escape variants carrying these mutations, respectively. Neither mAb neutralized later nAb escape or heterologous Envs. Crystal structures of the antigen-binding fragments (Fabs) revealed flat epitope contact surfaces, where minimal light chain mutation in 19.3H-L3 allowed for additional antigenic interactions. Resistance to mAb neutralization arose in later Envs through alteration of two glycans spatially adjacent to the initial escape signatures. The cross-neutralizing nAbs that ultimately developed failed to target any of the defined V3-proximal changes generated during the first year of infection in this subject. Our data demonstrate that this subject's first recognized nAb epitope elicited strain-specific mAbs, which incrementally acquired autologous breadth, and directed later B cell responses to target distinct portions of Env. This immune re-focusing could have triggered the evolution of cross-clade antibodies and suggests that exposure to a specific sequence of immune escape variants might promote broad humoral responses during HIV-1 infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI050409/AI/NIAID NIH HHS/United States
- P01 AI088610/AI/NIAID NIH HHS/United States
- R01 AI058706/AI/NIAID NIH HHS/United States
- R01 AI064060/AI/NIAID NIH HHS/United States
- P01 AI082274/AI/NIAID NIH HHS/United States
- P01-AI082274/AI/NIAID NIH HHS/United States
- R01-AI058706/AI/NIAID NIH HHS/United States
- P30-AI050409/AI/NIAID NIH HHS/United States
- R37 AI051231/AI/NIAID NIH HHS/United States
- P01-AI088610/AI/NIAID NIH HHS/United States
- R37-AI051231/AI/NIAID NIH HHS/United States
- R01-AI064060/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

