Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia
- PMID: 23468627
- PMCID: PMC3585175
- DOI: 10.1371/journal.ppat.1003188
Macrophage-expressed IFN-β contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia
Erratum in
-
Correction: Macrophage-expressed IFN-β Contributes to Apoptotic Alveolar Epithelial Cell Injury in Severe Influenza Virus Pneumonia.PLoS Pathog. 2016 Jun 15;12(6):e1005716. doi: 10.1371/journal.ppat.1005716. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27304804 Free PMC article.
Abstract
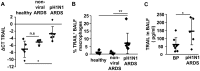
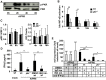
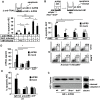
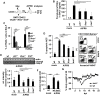
Influenza viruses (IV) cause pneumonia in humans with progression to lung failure and fatal outcome. Dysregulated release of cytokines including type I interferons (IFNs) has been attributed a crucial role in immune-mediated pulmonary injury during severe IV infection. Using ex vivo and in vivo IV infection models, we demonstrate that alveolar macrophage (AM)-expressed IFN-β significantly contributes to IV-induced alveolar epithelial cell (AEC) injury by autocrine induction of the pro-apoptotic factor TNF-related apoptosis-inducing ligand (TRAIL). Of note, TRAIL was highly upregulated in and released from AM of patients with pandemic H1N1 IV-induced acute lung injury. Elucidating the cell-specific underlying signalling pathways revealed that IV infection induced IFN-β release in AM in a protein kinase R- (PKR-) and NF-κB-dependent way. Bone marrow chimeric mice lacking these signalling mediators in resident and lung-recruited AM and mice subjected to alveolar neutralization of IFN-β and TRAIL displayed reduced alveolar epithelial cell apoptosis and attenuated lung injury during severe IV pneumonia. Together, we demonstrate that macrophage-released type I IFNs, apart from their well-known anti-viral properties, contribute to IV-induced AEC damage and lung injury by autocrine induction of the pro-apoptotic factor TRAIL. Our data suggest that therapeutic targeting of the macrophage IFN-β-TRAIL axis might represent a promising strategy to attenuate IV-induced acute lung injury.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, et al. (2009) Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med 361: 1935–1944. - PubMed
-
- Mauad T, Hajjar LA, Callegari GD, da Silva LF, Schout D, et al. (2010) Lung pathology in fatal novel human influenza A (H1N1) infection. Am J Respir Crit Care Med 181: 72–79. - PubMed
-
- Bem RA, Bos AP, Wosten-van Asperen RM, Bruijn M, Lutter R, et al. (2009) Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am J Respir Cell Mol Biol 42: 697–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

