FAM20A mutations can cause enamel-renal syndrome (ERS)
- PMID: 23468644
- PMCID: PMC3585120
- DOI: 10.1371/journal.pgen.1003302
FAM20A mutations can cause enamel-renal syndrome (ERS)
Abstract
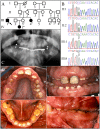
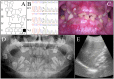
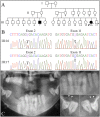
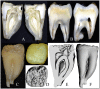
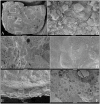
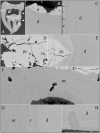
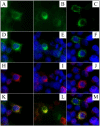
Enamel-renal syndrome (ERS) is an autosomal recessive disorder characterized by severe enamel hypoplasia, failed tooth eruption, intrapulpal calcifications, enlarged gingiva, and nephrocalcinosis. Recently, mutations in FAM20A were reported to cause amelogenesis imperfecta and gingival fibromatosis syndrome (AIGFS), which closely resembles ERS except for the renal calcifications. We characterized three families with AIGFS and identified, in each case, recessive FAM20A mutations: family 1 (c.992G>A; g.63853G>A; p.Gly331Asp), family 2 (c.720-2A>G; g.62232A>G; p.Gln241_Arg271del), and family 3 (c.406C>T; g.50213C>T; p.Arg136* and c.1432C>T; g.68284C>T; p.Arg478*). Significantly, a kidney ultrasound of the family 2 proband revealed nephrocalcinosis, revising the diagnosis from AIGFS to ERS. By characterizing teeth extracted from the family 3 proband, we demonstrated that FAM20A(-/-) molars lacked true enamel, showed extensive crown and root resorption, hypercementosis, and partial replacement of resorbed mineral with bone or coalesced mineral spheres. Supported by the observation of severe ectopic calcifications in the kidneys of Fam20a null mice, we conclude that FAM20A, which has a kinase homology domain and localizes to the Golgi, is a putative Golgi kinase that plays a significant role in the regulation of biomineralization processes, and that mutations in FAM20A cause both AIGFS and ERS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Pathognomonic oral profile of Enamel Renal Syndrome (ERS) caused by recessive FAM20A mutations.Orphanet J Rare Dis. 2014 Jun 14;9:84. doi: 10.1186/1750-1172-9-84. Orphanet J Rare Dis. 2014. PMID: 24927635 Free PMC article. Review.
-
FAM20A mutations and transcriptome analyses of dental pulp tissues of enamel renal syndrome.Int Endod J. 2023 Aug;56(8):943-954. doi: 10.1111/iej.13928. Epub 2023 May 16. Int Endod J. 2023. PMID: 37159186 Free PMC article.
-
Enamel-renal-gingival syndrome and FAM20A mutations.Am J Med Genet A. 2014 Jan;164A(1):1-9. doi: 10.1002/ajmg.a.36187. Epub 2013 Nov 20. Am J Med Genet A. 2014. PMID: 24259279
-
Pathogenesis of Enamel-Renal Syndrome Associated Gingival Fibromatosis: A Proteomic Approach.Front Endocrinol (Lausanne). 2021 Oct 29;12:752568. doi: 10.3389/fendo.2021.752568. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34777248 Free PMC article.
-
Two new families with enamel renal syndrome: A novel FAM20A gene mutation and review of literature.Eur J Med Genet. 2020 Nov;63(11):104045. doi: 10.1016/j.ejmg.2020.104045. Epub 2020 Aug 22. Eur J Med Genet. 2020. PMID: 32835847 Review.
Cited by
-
A secretory kinase complex regulates extracellular protein phosphorylation.Elife. 2015 Mar 19;4:e06120. doi: 10.7554/eLife.06120. Elife. 2015. PMID: 25789606 Free PMC article.
-
FAM20A mutations associated with enamel renal syndrome.J Dent Res. 2014 Jan;93(1):42-8. doi: 10.1177/0022034513512653. Epub 2013 Nov 6. J Dent Res. 2014. PMID: 24196488 Free PMC article.
-
Pathognomonic oral profile of Enamel Renal Syndrome (ERS) caused by recessive FAM20A mutations.Orphanet J Rare Dis. 2014 Jun 14;9:84. doi: 10.1186/1750-1172-9-84. Orphanet J Rare Dis. 2014. PMID: 24927635 Free PMC article. Review.
-
Regulation of secretory pathway kinase or kinase-like proteins in human cancers.Front Immunol. 2023 Feb 7;14:942849. doi: 10.3389/fimmu.2023.942849. eCollection 2023. Front Immunol. 2023. PMID: 36825005 Free PMC article. Review.
-
Recessive COL17A1 Mutations and a Dominant LAMB3 Mutation Cause Hypoplastic Amelogenesis Imperfecta.J Pers Med. 2023 Oct 14;13(10):1494. doi: 10.3390/jpm13101494. J Pers Med. 2023. PMID: 37888105 Free PMC article.
References
-
- MacGibbon D (1972) Generalized enamel hypoplasia and renal dysfunction. Aust Dent J 17: 61–63. - PubMed
-
- Lubinsky M, Angle C, Marsh PW, Witkop CJ Jr (1985) Syndrome of amelogenesis imperfecta, nephrocalcinosis, impaired renal concentration, and possible abnormality of calcium metabolism. Am J Med Genet 20: 233–243. - PubMed
-
- Hall RK, Phakey P, Palamara J, McCredie DA (1995) Amelogenesis imperfecta and nephrocalcinosis syndrome. Case studies of clinical features and ultrastructure of tooth enamel in two siblings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79: 583–592. - PubMed
-
- Normand de la Tranchade I, Bonarek H, Marteau JM, Boileau MJ, Nancy J (2003) Amelogenesis imperfecta and nephrocalcinosis: a new case of this rare syndrome. J Clin Pediatr Dent 27: 171–175. - PubMed
-
- Paula LM, Melo NS, Silva Guerra EN, Mestrinho DH, Acevedo AC (2005) Case report of a rare syndrome associating amelogenesis imperfecta and nephrocalcinosis in a consanguineous family. Arch Oral Biol 50: 237–242. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

