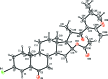
(25R)-16β-Acet-oxy-3β-bromo-23',26-ep-oxy-23',25-dimethyl-5α-cholest-23,23'-en-6-one dichloro-methane monosolvate
- PMID: 23468778
- PMCID: PMC3588813
- DOI: 10.1107/S1600536812043590
(25R)-16β-Acet-oxy-3β-bromo-23',26-ep-oxy-23',25-dimethyl-5α-cholest-23,23'-en-6-one dichloro-methane monosolvate
Abstract
The crystal structure of the title compound, C31H45BrO5·CH2Cl2, prepared in six steps from diosgenin, confirmed that the configurations of the stereogenic centers, positions 20S and 25R, remain unchanged during the reaction. The six-membered A, B and C rings have chair conformations. The five-membered ring D has an envelope conformation (with the methyl-substituted C atom fused to ring C as the flap) and the six-membered dihydro-pyran ring E adopts a twist-boat conformation. In the crystal, mol-ecules are linked via C-H⋯O and C-H⋯Cl hydrogen bonds, the latter involving the dichloro-methane solvent mol-ecule, forming a three-dimensional supra-molecular network.
Figures
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2. pp. S1–19.
-
- Castro-Méndez, A., Sandoval-Ramírez, J. & Bernès, S. (2002). Acta Cryst. E58, o606–o608.
-
- Corey, E. J. & Suggs, J. W. (1975). Tetrahedron Lett. 16, 2647–2650.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials