3-[(Furan-2-yl)carbon-yl]-1-(pyrimi-din-2-yl)thio-urea
- PMID: 23468808
- PMCID: PMC3588843
- DOI: 10.1107/S1600536812044029
3-[(Furan-2-yl)carbon-yl]-1-(pyrimi-din-2-yl)thio-urea
Abstract
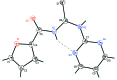
The title compound, C10H8N4O2S, was synthesized from furoyl isothio-cynate and 2-amino-pyrimidine in dry acetone. The two N-H groups are in an anti conformation with respect to each other and one N-H group is anti to the C=S group while the other is syn. The amide C=S and the C=O groups are syn to each other. The mean plane of the central thio-urea fragment forms dihedral angles of 13.50 (14) and 5.03 (11)° with the furan and pyrimidine rings, respectively. The dihedral angle between the furan and pyrimidine rings is 18.43 (10)°. The mol-ecular conformation is stabilized by an intra-molecular N-H⋯N hydrogen bond generating an S(6) ring motif. In the crystal, mol-ecules are linked by pairs of N-H⋯N and weak C-H⋯S hydrogen bonds to form inversion dimers.
Figures
References
-
- Agilent (2011). CrysAlis PRO. Agilent Technologies, Yarnton, Oxfordshire, England.
-
- Aly, A. A., Ahmed, E. K., El-Mokadem, K. M. & Hegazy, M. E. F. (2007). J. Sulfur Chem. 28, 73–93.
-
- D’hooghe, M., Waterinckx, A. & De Kimpe, N. (2005). J. Org. Chem. 70, 227–232. - PubMed
-
- Duque, J., Estevez-Hernandez, O., Reguera, E., Ellena, J. & Correa, R. S. (2009). J. Coord. Chem. 62, 2804–2813.
-
- Hassan, N. N. N., Kadir, M. A., Yusof, M. S. M. & Yamin, B. M. (2007). Acta Cryst. E63, o4224.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
