The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies
- PMID: 23468921
- PMCID: PMC3584122
- DOI: 10.1371/journal.pone.0057121
The association between serum biomarkers and disease outcome in influenza A(H1N1)pdm09 virus infection: results of two international observational cohort studies
Abstract
Background: Prospective studies establishing the temporal relationship between the degree of inflammation and human influenza disease progression are scarce. To assess predictors of disease progression among patients with influenza A(H1N1)pdm09 infection, 25 inflammatory biomarkers measured at enrollment were analyzed in two international observational cohort studies.
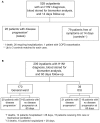
Methods: Among patients with RT-PCR-confirmed influenza A(H1N1)pdm09 virus infection, odds ratios (ORs) estimated by logistic regression were used to summarize the associations of biomarkers measured at enrollment with worsened disease outcome or death after 14 days of follow-up for those seeking outpatient care (FLU 002) or after 60 days for those hospitalized with influenza complications (FLU 003). Biomarkers that were significantly associated with progression in both studies (p<0.05) or only in one (p<0.002 after Bonferroni correction) were identified.
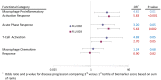
Results: In FLU 002 28/528 (5.3%) outpatients had influenza A(H1N1)pdm09 virus infection that progressed to a study endpoint of complications, hospitalization or death, whereas in FLU 003 28/170 (16.5%) inpatients enrolled from the general ward and 21/39 (53.8%) inpatients enrolled directly from the ICU experienced disease progression. Higher levels of 12 of the 25 markers were significantly associated with subsequent disease progression. Of these, 7 markers (IL-6, CD163, IL-10, LBP, IL-2, MCP-1, and IP-10), all with ORs for the 3(rd) versus 1(st) tertile of 2.5 or greater, were significant (p<0.05) in both outpatients and inpatients. In contrast, five markers (sICAM-1, IL-8, TNF-α, D-dimer, and sVCAM-1), all with ORs for the 3(rd) versus 1(st) tertile greater than 3.2, were significantly (p≤.002) associated with disease progression among hospitalized patients only.
Conclusions: In patients presenting with varying severities of influenza A(H1N1)pdm09 virus infection, a baseline elevation in several biomarkers associated with inflammation, coagulation, or immune function strongly predicted a higher risk of disease progression. It is conceivable that interventions designed to abrogate these baseline elevations might affect disease outcome.
Conflict of interest statement
Figures


References
-
- Centers for Disease Control and Prevention Outbreak of swine-origin influenza A (H1N1) virus infection – Mexico, March–April 2009. (2009) MMWR Morb Mortal Wkly Rep 58: : 467–470. - PubMed
-
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team (2009) Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360: 2605–2615. - PubMed
-
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza (2010) Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 362: 1708–19. - PubMed
-
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, et al. (2009) Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 361: 1935–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

