EM structure of the ectodomain of integrin CD11b/CD18 and localization of its ligand-binding site relative to the plasma membrane
- PMID: 23469114
- PMCID: PMC3585415
- DOI: 10.1371/journal.pone.0057951
EM structure of the ectodomain of integrin CD11b/CD18 and localization of its ligand-binding site relative to the plasma membrane
Abstract
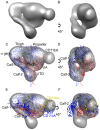
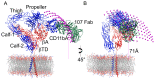
One-half of the integrin α-subunit Propeller domains contain and extra vWFA domain (αA domain), which mediates integrin binding to extracellular physiologic ligands via its metal-ion-dependent adhesion site (MIDAS). We used electron microscopy to determine the 3D structure of the αA-containing ectodomain of the leukocyte integrin CD11b/CD18 (αMβ2) in its inactive state. A well defined density for αA was observed within a bent ectodomain conformation, while the structure of the ectodomain in complex with the Fab fragment of mAb107, which binds at the MIDAS face of CD11b and stabilizes the inactive state, further revealed that αA is restricted to a relatively small range of orientations relative to the Propeller domain. Using Fab 107 as probe in fluorescent lifetime imaging microscopy (FLIM) revealed that αA is positioned relatively far from the membrane surface in the inactive state, and a systematic orientation search revealed that the MIDAS face would be accessible to extracellular ligand in the inactive state of the full-length cellular integrin. These studies are the first to define the 3D EM structure of an αA-containing integrin ectodomain and to position the ligand-binding face of αA domain in relation to the plasma membrane, providing new insights into current models of integrin activation.
Conflict of interest statement
Figures



References
-
- Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687. - PubMed
-
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, et al. (2002) Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296: 151–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

