A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS
- PMID: 23469212
- PMCID: PMC3585978
- DOI: 10.1371/journal.pone.0057634
A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS
Abstract
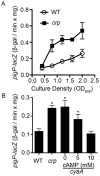
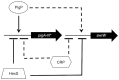
Swarming motility and hemolysis are virulence-associated determinants for a wide array of pathogenic bacteria. The broad host-range opportunistic pathogen Serratia marcescens produces serratamolide, a small cyclic amino-lipid, that promotes swarming motility and hemolysis. Serratamolide is negatively regulated by the transcription factors HexS and CRP. Positive regulators of serratamolide production are unknown. Similar to serratamolide, the antibiotic pigment, prodigiosin, is regulated by temperature, growth phase, HexS, and CRP. Because of this co-regulation, we tested the hypothesis that a homolog of the PigP transcription factor of the atypical Serratia species ATCC 39006, which positively regulates prodigiosin biosynthesis, is also a positive regulator of serratamolide production in S. marcescens. Mutation of pigP in clinical, environmental, and laboratory strains of S. marcescens conferred pleiotropic phenotypes including the loss of swarming motility, hemolysis, and severely reduced prodigiosin and serratamolide synthesis. Transcriptional analysis and electrophoretic mobility shift assays place PigP in a regulatory pathway with upstream regulators CRP and HexS. The data from this study identifies a positive regulator of serratamolide production, describes novel roles for the PigP transcription factor, shows for the first time that PigP directly regulates the pigment biosynthetic operon, and identifies upstream regulators of pigP. This study suggests that PigP is important for the ability of S. marcescens to compete in the environment.
Conflict of interest statement
Figures







References
-
- Diekema DJ, Pfaller MA, Jones RN, Doern GV, Winokur PL, et al. (1999) Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis 29: 595–607. - PubMed
-
- Richards MJ, Edwards JR, Culver DH, Gaynes RP (2000) Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 21: 510–515. - PubMed
-
- Jones RN (2010) Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 51 Suppl 1S81–87. - PubMed
-
- Verhamme KM, De Coster W, De Roo L, De Beenhouwer H, Nollet G, et al. (2007) Pathogens in early-onset and late-onset intensive care unit-acquired pneumonia. Infect Control Hosp Epidemiol 28: 389–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

