A new oxidative stress model, 2,2-azobis(2-amidinopropane) dihydrochloride induces cardiovascular damages in chicken embryo
- PMID: 23469224
- PMCID: PMC3585800
- DOI: 10.1371/journal.pone.0057732
A new oxidative stress model, 2,2-azobis(2-amidinopropane) dihydrochloride induces cardiovascular damages in chicken embryo
Abstract
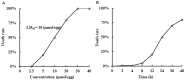
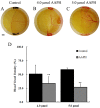
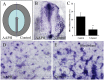
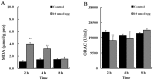
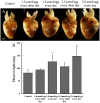
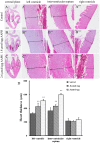
It is now well established that the developing embryo is very sensitive to oxidative stress, which is a contributing factor to pregnancy-related disorders. However, little is known about the effects of reactive oxygen species (ROS) on the embryonic cardiovascular system due to a lack of appropriate ROS control method in the placenta. In this study, a small molecule called 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH), a free radicals generator, was used to study the effects of oxidative stress on the cardiovascular system during chick embryo development. When nine-day-old (stage HH 35) chick embryos were treated with different concentrations of AAPH inside the air chamber, it was established that the LD50 value for AAPH was 10 µmol/egg. At this concentration, AAPH was found to significantly reduce the density of blood vessel plexus that was developed in the chorioallantoic membrane (CAM) of HH 35 chick embryos. Impacts of AAPH on younger embryos were also examined and discovered that it inhibited the development of vascular plexus on yolk sac in HH 18 embryos. AAPH also dramatically repressed the development of blood islands in HH 3+ embryos. These results implied that AAPH-induced oxidative stress could impair the whole developmental processes associated with vasculogenesis and angiogenesis. Furthermore, we observed heart enlargement in the HH 40 embryo following AAPH treatment, where the left ventricle and interventricular septum were found to be thickened in a dose-dependent manner due to myocardiac cell hypertrophy. In conclusion, oxidative stress, induced by AAPH, could lead to damage of the cardiovascular system in the developing chick embryo. The current study also provided a new developmental model, as an alternative for animal and cell models, for testing small molecules and drugs that have anti-oxidative activities.
Conflict of interest statement
Figures


References
-
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84. - PubMed
-
- Abramov JP, Wells PG (2012) Embryoprotective role of endogenous catalase in acatalasemic and human catalase-expressing mouse embryos exposed in culture to developmental and phenytoin-enhanced oxidative stress. Toxicol Sci 120: 428–438. - PubMed
-
- Wan J, Winn LM (2006) In utero–initiated cancer: The role of reactive oxygen species. Birth Defects Res C Embryo Today 78: 326–332. - PubMed
-
- Dennery PA (2007) Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today 81: 155–162. - PubMed
-
- Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, Castro-Obregón S (2008) Function of reactive oxygen species during animal development: Passive or active? Dev Biol 320: 1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

