Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials
- PMID: 23469246
- PMCID: PMC3585808
- DOI: 10.1371/journal.pone.0057838
Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials
Abstract
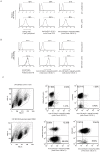
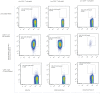
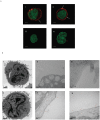
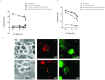
Clinical trials targeting CD19 on B-cell malignancies are underway with encouraging anti-tumor responses. Most infuse T cells genetically modified to express a chimeric antigen receptor (CAR) with specificity derived from the scFv region of a CD19-specific mouse monoclonal antibody (mAb, clone FMC63). We describe a novel anti-idiotype monoclonal antibody (mAb) to detect CD19-specific CAR(+) T cells before and after their adoptive transfer. This mouse mAb was generated by immunizing with a cellular vaccine expressing the antigen-recognition domain of FMC63. The specificity of the mAb (clone no. 136.20.1) was confined to the scFv region of the CAR as validated by inhibiting CAR-dependent lysis of CD19(+) tumor targets. This clone can be used to detect CD19-specific CAR(+) T cells in peripheral blood mononuclear cells at a sensitivity of 1∶1,000. In clinical settings the mAb is used to inform on the immunophenotype and persistence of administered CD19-specific T cells. Thus, our CD19-specific CAR mAb (clone no. 136.20.1) will be useful to investigators implementing CD19-specific CAR(+) T cells to treat B-lineage malignancies. The methodology described to develop a CAR-specific anti-idiotypic mAb could be extended to other gene therapy trials targeting different tumor associated antigens in the context of CAR-based adoptive T-cell therapy.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

