Kinetic and spectroscopic studies of bicupin oxalate oxidase and putative active site mutants
- PMID: 23469254
- PMCID: PMC3585803
- DOI: 10.1371/journal.pone.0057933
Kinetic and spectroscopic studies of bicupin oxalate oxidase and putative active site mutants
Abstract
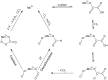
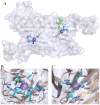
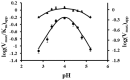
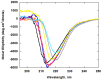
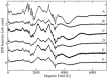
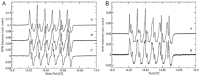
Ceriporiopsis subvermispora oxalate oxidase (CsOxOx) is the first bicupin enzyme identified that catalyzes manganese-dependent oxidation of oxalate. In previous work, we have shown that the dominant contribution to catalysis comes from the monoprotonated form of oxalate binding to a form of the enzyme in which an active site carboxylic acid residue must be unprotonated. CsOxOx shares greatest sequence homology with bicupin microbial oxalate decarboxylases (OxDC) and the 241-244DASN region of the N-terminal Mn binding domain of CsOxOx is analogous to the lid region of OxDC that has been shown to determine reaction specificity. We have prepared a series of CsOxOx mutants to probe this region and to identify the carboxylate residue implicated in catalysis. The pH profile of the D241A CsOxOx mutant suggests that the protonation state of aspartic acid 241 is mechanistically significant and that catalysis takes place at the N-terminal Mn binding site. The observation that the D241S CsOxOx mutation eliminates Mn binding to both the N- and C- terminal Mn binding sites suggests that both sites must be intact for Mn incorporation into either site. The introduction of a proton donor into the N-terminal Mn binding site (CsOxOx A242E mutant) does not affect reaction specificity. Mutation of conserved arginine residues further support that catalysis takes place at the N-terminal Mn binding site and that both sites must be intact for Mn incorporation into either site.
Conflict of interest statement
Figures
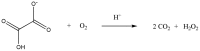

References
-
- Kotsira VP, Clonis YD (1997) Oxalate oxidase from barley roots: purification to homogeneity and study of some molecular, catalytic, and binding properties. Arch Biochem Biophys 340: 239–249. - PubMed
-
- Whittaker MM, Whittaker JW (2002) Characterization of recombinant barley oxalate oxidase expressed by Pichia pastoris. J Biol Inorg Chem 7: 136–145. - PubMed
-
- Hesse A, Bongartz H, Heynck H, Berg W (1996) Measurement of urinary oxalic acid: a comparison of five methods. Clin Biochem 29: 467–472. - PubMed
-
- Honow R, Bongartz D, Hesse A (1997) An improved HPLC-enzyme-reactor method for the determination of oxalic acid in complex matrices. Clin Chim Acta 261: 131–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

