Differential expression of Toll-like receptors in dendritic cells of patients with dengue during early and late acute phases of the disease
- PMID: 23469297
- PMCID: PMC3585035
- DOI: 10.1371/journal.pntd.0002060
Differential expression of Toll-like receptors in dendritic cells of patients with dengue during early and late acute phases of the disease
Abstract
Background: Dengue hemorrhagic fever (DHF) is observed in individuals that have pre-existing heterotypic dengue antibodies and is associated with increased viral load and high levels of pro-inflammatory cytokines early in infection. Interestingly, a recent study showed that dengue virus infection in the presence of antibodies resulted in poor stimulation of Toll-like receptors (TLRs), thereby facilitating virus particle production, and also suggesting that TLRs may contribute to disease pathogenesis.
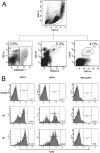
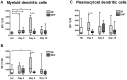
Methodology/principal findings: We evaluated the expression levels of TLR2, 3, 4 and 9 and the co-stimulatory molecules CD80 and CD86 by flow cytometry. This was evaluated in monocytes, in myeloid and plasmacytoid dendritic cells (mDCs and pDCs) from 30 dengue patients with different clinical outcomes and in 20 healthy controls. Increased expression of TLR3 and TLR9 in DCs of patients with dengue fever (DF) early in infection was detected. In DCs from patients with severe manifestations, poor stimulation of TLR3 and TLR9 was observed. In addition, we found a lower expression of TLR2 in patients with DF compared to DHF. Expression levels of TLR4 were not affected. Furthermore, the expression of CD80 and CD86 was altered in mDCs and CD86 in pDCs of severe dengue cases. We show that interferon alpha production decreased in the presence of dengue virus after stimulation of PBMCs with the TLR9 agonist (CpG A). This suggests that the virus can affect the interferon response through this signaling pathway.
Conclusions/significance: These results show that during dengue disease progression, the expression profile of TLRs changes depending on the severity of the disease. Changes in TLRs expression could play a central role in DC activation, thereby influencing the innate immune response.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures





References
-
- WHO (2009) Dengue and Dengue Hemorrhagic Fever. World Health Organization.
-
- Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. Adv Virus Res 59: 23–61. - PubMed
-
- WHO (1997) Dengue Hemorrhagic fever: Diagnosis, treatment, prevention and control. World Health Organization.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

