New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis
- PMID: 23470494
- PMCID: PMC3675388
- DOI: 10.1093/eurheartj/eht049
New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis
Abstract
Background: Oral anticoagulation in addition to antiplatelet treatment after an acute coronary syndrome might reduce ischaemic events but increase bleeding risk. We performed a meta-analysis to evaluate the efficacy and safety of adding direct thrombin or factor-Xa inhibition by any of the novel oral anticoagulants (apixaban, dabigatran, darexaban, rivaroxaban, and ximelagatran) to single (aspirin) or dual (aspirin and clopidogrel) antiplatelet therapy in this setting.
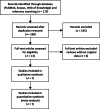
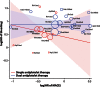
Methods and results: All seven published randomized, placebo-controlled phase II and III studies of novel oral anticoagulants in acute coronary syndromes were included. The database consisted of 30 866 patients, 4135 (13.4%) on single, and 26 731 (86.6%) on dual antiplatelet therapy, with a non-ST- or ST-elevation acute coronary syndrome within the last 7-14 days. We defined major adverse cardiovascular events (MACEs) as the composite of all-cause mortality, myocardial infarction, or stroke; and clinically significant bleeding as the composite of major and non-major bleeding requiring medical attention according to the study definitions. When compared with aspirin alone the combination of an oral anticoagulant and aspirin reduced the incidence of MACE [hazard ratio (HR) and 95% confidence interval 0.70; 0.59-0.84], but increased clinically significant bleeding (HR: 1.79; 1.54-2.09). Compared with dual antiplatelet therapy with aspirin and clopidogrel, adding an oral anticoagulant decreased the incidence of MACE modestly (HR: 0.87; 0.80-0.95), but more than doubled the bleeding (HR: 2.34; 2.06-2.66). Heterogeneity between studies was low, and results were similar when restricting the analysis to phase III studies.
Conclusion: In patients with a recent acute coronary syndrome, the addition of a new oral anticoagulant to antiplatelet therapy results in a modest reduction in cardiovascular events but a substantial increase in bleeding, most pronounced when new oral anticoagulants are combined with dual antiplatelet therapy.
Keywords: Acute coronary syndrome; Antiplatelet therapy; Meta-analysis; Myocardial infarction; Oral anticoagulants.
Figures



Comment in
-
Combined antiplatelet and novel oral anticoagulant therapy after acute coronary syndrome: is three a crowd?Eur Heart J. 2013 Jun;34(22):1618-20. doi: 10.1093/eurheartj/eht075. Epub 2013 Mar 12. Eur Heart J. 2013. PMID: 23482521 No abstract available.
-
ACP Journal Club. Review: New oral anticoagulants added to antiplatelets reduce CV events but increase bleeding after ACS.Ann Intern Med. 2013 Jul 16;159(2):JC7. doi: 10.7326/0003-4819-159-2-201307160-02007. Ann Intern Med. 2013. PMID: 23856705 No abstract available.
References
-
- Steg PG, James SK, Atar D, Badano LP, Blomström-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–2619. - PubMed
-
- Kushner FG, Hand M, Smith SC, Jr, King SB, III, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Circulation. 2009;120:2271–2306. - PubMed
-
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. - PubMed
-
- Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Jneid H, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Jacobs AK. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/ non-ST-elevation myocardial infarction. Circulation. 2011;123:2022–2060. - PubMed
-
- Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

