The loss of the BH3-only Bcl-2 family member Bid delays T-cell leukemogenesis in Atm-/- mice
- PMID: 23470523
- PMCID: PMC3679453
- DOI: 10.1038/cdd.2013.16
The loss of the BH3-only Bcl-2 family member Bid delays T-cell leukemogenesis in Atm-/- mice
Abstract
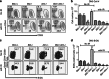
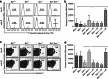
Multicellular organisms maintain genomic integrity and resist tumorigenesis through a tightly regulated DNA damage response (DDR) that prevents propagation of deleterious mutations either through DNA repair or programmed cell death. An impaired DDR leads to tumorigenesis that is accelerated when programmed cell death is prevented. Loss of the ATM (ataxia telangiectasia mutated)-mediated DDR in mice results in T-cell leukemia driven by accumulation of DNA damage accrued during normal T-cell development. Pro-apoptotic BH3-only Bid is a substrate of Atm, and Bid phosphorylation is required for proper cell cycle checkpoint control and regulation of hematopoietic function. In this report, we demonstrate that, surprisingly, loss of Bid increases the latency of leukemogenesis in Atm-/- mice. Bid-/-Atm-/- mice display impaired checkpoint control and increased cell death of DN3 thymocytes. Loss of Bid thus inhibits T-cell tumorigenesis by increasing clearance of damaged cells, and preventing propagation of deleterious mutations.
Figures





Comment in
-
Genetic background and tumour susceptibility in mouse models.Cell Death Differ. 2013 Jul;20(7):964. doi: 10.1038/cdd.2013.35. Epub 2013 Apr 26. Cell Death Differ. 2013. PMID: 23618812 Free PMC article. No abstract available.
-
Bid-ding for mercy: twisted killer in action.Cell Death Differ. 2013 Jul;20(7):847-9. doi: 10.1038/cdd.2013.40. Cell Death Differ. 2013. PMID: 23749178 Free PMC article. No abstract available.
References
-
- Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Mol Cell. 2012;46:722–734. - PubMed
-
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. - PubMed
-
- Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5:792–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

