Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers
- PMID: 23470965
- PMCID: PMC3630270
- DOI: 10.1158/1078-0432.CCR-12-2246
Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers
Abstract
Purpose: All patients with EGF receptor (EGFR)-mutant lung cancers eventually develop acquired resistance to EGFR tyrosine kinase inhibitors (TKI). Smaller series have identified various mechanisms of resistance, but systematic evaluation of a large number of patients to definitively establish the frequency of various mechanisms has not been conducted.
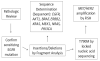
Experimental design: Patients with lung adenocarcinomas and acquired resistance to erlotinib or gefitinib enrolled onto a prospective biopsy protocol and underwent a rebiopsy after the development of acquired resistance. Histology was reviewed. Samples underwent genotyping for mutations in EGFR, AKT1, BRAF, ERBB2, KRAS, MEK1, NRAS and PIK3CA, and FISH for MET and HER2.
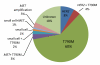
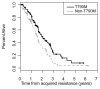
Results: Adequate tumor samples for molecular analysis were obtained in 155 patients. Ninety-eight had second-site EGFR T790M mutations [63%; 95% confidence interval (CI), 55%-70%] and four had small cell transformation (3%, 95% CI, 0%-6%). MET amplification was seen in 4 of 75 (5%; 95% CI, 1%-13%). HER2 amplification was seen in 3 of 24 (13%; 95% CI, 3%-32%). We did not detect any acquired mutations in PIK3CA, AKT1, BRAF, ERBB2, KRAS, MEK1, or NRAS (0 of 88, 0%; 95% CI, 0%-4%). Overlap among mechanisms of acquired resistance was seen in 4%.
Conclusions: This is the largest series reporting mechanisms of acquired resistance to EGFR-TKI therapy. We identified EGFR T790M as the most common mechanism of acquired resistance, whereas MET amplification, HER2 amplification, and small cell histologic transformation occur less frequently. More comprehensive methods to characterize molecular alterations in this setting are needed to improve our understanding of acquired resistance to EGFR-TKIs.
Figures


References
-
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. The lancet oncology. 2010;11:121–8. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. - PubMed
-
- Janne PA, Wang X, Socinski MA, Crawford J, Stinchcombe TE, Gu L, et al. Randomized Phase II Trial of Erlotinib Alone or With Carboplatin and Paclitaxel in Patients Who Were Never or Light Former Smokers With Advanced Lung Adenocarcinoma: CALGB 30406 Trial. J Clin Oncol. 2012;30(17):2063–9. - PMC - PubMed
-
- Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

