RNA elements directing in vivo assembly of the 7SK/MePCE/Larp7 transcriptional regulatory snRNP
- PMID: 23471002
- PMCID: PMC3632141
- DOI: 10.1093/nar/gkt159
RNA elements directing in vivo assembly of the 7SK/MePCE/Larp7 transcriptional regulatory snRNP
Abstract
Through controlling the nuclear level of active positive transcription elongation factor b (P-TEFb), the 7SK small nuclear RNA (snRNA) functions as a key regulator of RNA polymerase II transcription. Together with hexamethylene bisacetamide-inducible proteins 1/2 (HEXIM1/2), the 7SK snRNA sequesters P-TEFb into transcriptionally inactive ribonucleoprotein (RNP). In response to transcriptional stimulation, the 7SK/HEXIM/P-TEFb RNP releases P-TEFb to promote polymerase II-mediated messenger RNA synthesis. Besides transiently associating with HEXIM1/2 and P-TEFb, the 7SK snRNA stably interacts with the La-related protein 7 (Larp7) and the methylphosphate capping enzyme (MePCE). In this study, we used in vivo RNA-protein interaction assays to determine the sequence and structural elements of human 7SK snRNA directing assembly of the 7SK/MePCE/Larp7 core snRNP. MePCE interacts with the short 5'-terminal G1-U4/U106-G111 helix-tail motif and Larp7 binds to the 3'-terminal hairpin and the following U-rich tail of 7SK. The overall RNA structure and some particular nucleotides provide the information for specific binding of MePCE and Larp7. We also demonstrate that binding of Larp7 to 7SK is a prerequisite for in vivo recruitment of P-TEFb, indicating that besides providing stability for 7SK, Larp7 directly participates in P-TEFb regulation. Our results provide further explanation for the frequently observed link between Larp7 mutations and cancer development.
Figures


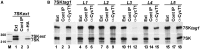
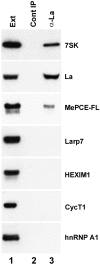
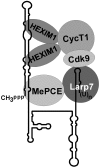
References
-
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol. Cell. 2006;23:297–305. - PubMed
-
- Yamaguchi Y, Takagi T, Wada T, Yano K, Furuya A, Sugimoto S, Hasegawa J, Handa H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell. 1999;97:41–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

