Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial
- PMID: 23471421
- PMCID: PMC3681929
- DOI: 10.1001/jamainternmed.2013.915
Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial
Abstract
Importance: Controversy remains about whether depression can be successfully managed after acute coronary syndrome (ACS) and the costs and benefits of doing so.
Objective: To determine the effects of providing post-ACS depression care on depressive symptoms and health care costs.
Design: Multicenter randomized controlled trial.
Setting: Patients were recruited from 2 private and 5 academic ambulatory centers across the United States.
Participants: A total of 150 patients with elevated depressive symptoms (Beck Depression Inventory [BDI] score ≥10) 2 to 6 months after an ACS, recruited between March 18, 2010, and January 9, 2012.
Interventions: Patients were randomized to 6 months of centralized depression care (patient preference for problem-solving treatment given via telephone or the Internet, pharmacotherapy, both, or neither), stepped every 6 to 8 weeks (active treatment group; n = 73), or to locally determined depression care after physician notification about the patient's depressive symptoms (usual care group; n = 77).
Main outcome measures: Change in depressive symptoms during 6 months and total health care costs.
Results: Depressive symptoms decreased significantly more in the active treatment group than in the usual care group (differential change between groups, -3.5 BDI points; 95% CI, -6.1 to -0.7; P = .01). Although mental health care estimated costs were higher for active treatment than for usual care, overall health care estimated costs were not significantly different (difference adjusting for confounding, -$325; 95% CI, -$2639 to $1989; P = .78).
Conclusions: For patients with post-ACS depression, active treatment had a substantial beneficial effect on depressive symptoms. This kind of depression care is feasible, effective, and may be cost-neutral within 6 months; therefore, it should be tested in a large phase 3 pragmatic trial.
Trial registration: clinicaltrials.gov Identifier: NCT01032018.
Figures
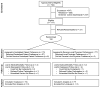

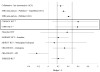
Comment in
-
Benefits and costs of improving depression treatment in people with heart disease: comment on "centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression".JAMA Intern Med. 2013 Jun 10;173(11):1004-5. doi: 10.1001/jamainternmed.2013.925. JAMA Intern Med. 2013. PMID: 23471438 No abstract available.
-
Centralised depression care according to patient preference is effective for the treatment of depression symptoms following acute coronary syndrome.Evid Based Ment Health. 2013 Nov;16(4):107. doi: 10.1136/eb-2013-101478. Epub 2013 Aug 21. Evid Based Ment Health. 2013. PMID: 23966129 No abstract available.
-
Exercise, cardiac rehabilitation, and post-acute coronary syndrome depression.JAMA Intern Med. 2014 Jan;174(1):165-6. doi: 10.1001/jamainternmed.2013.11112. JAMA Intern Med. 2014. PMID: 24394932 No abstract available.
-
Exercise, cardiac rehabilitation, and post-acute coronary syndrome depression--reply.JAMA Intern Med. 2014 Jan;174(1):166-7. doi: 10.1001/jamainternmed.2013.11097. JAMA Intern Med. 2014. PMID: 24394933 No abstract available.
References
-
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. - PubMed
-
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. - PubMed
-
- Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–1053. - PubMed
-
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. - PubMed
-
- Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 Suppl 2):S20–27. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

