Diagnostic imaging and biopsy pathways following abnormal screen-film and digital screening mammography
- PMID: 23471650
- PMCID: PMC3640408
- DOI: 10.1007/s10549-013-2466-5
Diagnostic imaging and biopsy pathways following abnormal screen-film and digital screening mammography
Abstract
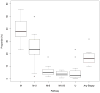
The transition from screen-film to digital mammography may have altered diagnostic evaluation of women following a positive screening examination. This study compared the use and timeliness of diagnostic imaging and biopsy for women screened with screen-film or digital mammography. Data were obtained from 35,321 positive screening mammograms on 32,087 women aged 40-89 years, from 22 breast cancer surveillance consortium facilities in 2005-2008. Diagnostic pathways were classified by their inclusion of diagnostic mammography, ultrasound, magnetic resonance imaging, and biopsy. We compared time to resolution and frequency of diagnostic pathways by patient characteristics, screening exam modality, and radiology facility. Between-facility differences were evaluated by computing the proportion of mammograms receiving follow-up with a particular pathway for each facility and examining variation in these proportions across facilities. Multinomial logistic regression adjusting for age, calendar year, and facility compared odds of follow-up with each pathway. The median time to resolution of a positive screening mammogram was 10 days. Compared to screen-film mammograms, digital mammograms were more frequently followed by only a single diagnostic mammogram (46 vs. 36 %). Pathways following digital screening mammography were also less likely to include biopsy (16 vs. 20 %). However, in adjusted analyses, most differences were not statistically significant (p = 0.857 for mammography only; p = 0.03 for biopsy). Substantial variability in diagnostic pathway frequency was seen across facilities. For instance, the frequency of evaluation with diagnostic mammography alone ranged from 23 to 55 % across facilities. Differences in evaluation of positive digital and screen-film screening mammograms were minor, and appeared to be largely attributable to substantial variation between radiology facilities. To guide health systems in their efforts to eliminate practices that do not contribute to effective care, we need further research to identify the causes of this variation and the best evidence-based approach for follow-up.
Figures
References
-
- Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2002;137:347– 360. - PubMed
-
- Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiologic Clinics of North America. 2004;42:793– 806. - PubMed
-
- Newman L. IOM report sets policy priorities for improving breast cancer screening. J Natl Cancer Inst. 2001;93 (8):574–575. - PubMed
-
- FDA. [Accessed February 11, 2013.];MQSA National Statistics. Available from: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandard....
Publication types
MeSH terms
Grants and funding
- U01 CA063740/CA/NCI NIH HHS/United States
- U01CA69976/CA/NCI NIH HHS/United States
- U01 CA070040/CA/NCI NIH HHS/United States
- U01CA70013/CA/NCI NIH HHS/United States
- U01 CA086082/CA/NCI NIH HHS/United States
- P01 CA154292/CA/NCI NIH HHS/United States
- 261201100031C/PHS HHS/United States
- U01CA63736/CA/NCI NIH HHS/United States
- U01CA86082/CA/NCI NIH HHS/United States
- HHSN261201100031C/CA/NCI NIH HHS/United States
- U01CA70040/CA/NCI NIH HHS/United States
- U01CA63740/CA/NCI NIH HHS/United States
- U01 CA063731/CA/NCI NIH HHS/United States
- U01 CA086076/CA/NCI NIH HHS/United States
- U01 CA069976/CA/NCI NIH HHS/United States
- U01CA86076/CA/NCI NIH HHS/United States
- U01 CA063736/CA/NCI NIH HHS/United States
- U01 CA070013/CA/NCI NIH HHS/United States
- U01CA63731/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

