Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India
- PMID: 23472135
- PMCID: PMC3589473
- DOI: 10.1371/journal.pone.0058080
Comparative evaluation of bivalent malaria rapid diagnostic tests versus traditional methods in field with special reference to heat stability testing in Central India
Abstract
Background: Malaria presents a diagnostic challenge in areas where both Plasmodium falciparum and P.vivax are co-endemic. Bivalent Rapid Diagnostic tests (RDTs) showed promise as diagnostic tools for P.falciparum and P.vivax. To assist national malaria control programme in the selection of RDTs, commercially available seven malaria RDTs were evaluated in terms of their performance with special reference to heat stability.
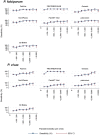
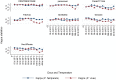
Methodology/principal findings: This study was undertaken in four forested districts of central India (July, 2011- March, 2012). All RDTs were tested simultaneously in field along with microscopy as gold standard. These RDTs were stored in their original packing at 25°C before transport to the field or they were stored at 35°C and 45°C upto 100 days for testing the performance of RDTs at high temperature. In all 2841 patients with fever were screened for malaria of which 26% were positive for P.falciparum, and 17% for P.vivax. The highest sensitivity of any RDT for P.falciparum was 98% (95% CI; 95.9-98.8) and lowest sensitivity was 76% (95% CI; 71.7-79.6). For P.vivax highest and lowest sensitivity for any RDT was 80% (95% CI; 94.9 - 83.9) and 20% (95% CI; 15.6-24.5) respectively. Heat stability experiments showed that most RDTs for P.falciparum showed high sensitivity at 45°C upto 90 days. While for P.vivax only two RDTs maintained good sensitivity upto day 90 when compared with RDTs kept at room temperature. Agreement between observers was excellent for positive and negative readings for both P.falciparum and P.vivax (Kappa >0.6-0.9).
Conclusion: This is first field evaluation of RDTs regarding their temperature stability. Although RDTs are useful as diagnostic tool for P.falciparum and P.vivax even at high temperature, the quality of RDTs should be regulated and monitored more closely.
Conflict of interest statement
Figures
References
-
- Singh N, Nagpal AC (2004) Performance of the OptiMAL dipstick test for management of severe and complicated malaria cases in a tertiary hospital, central India. J Infect 48: 364–365. - PubMed
-
- Marsh K, English M, Peshu N, Crawley J, Snow R (1996) Clinical algorithm for malaria in Africa. Lancet 347: 1327–1328. - PubMed
-
- World Health Organization (2009) Malaria Microscopy Quality Assurance Manual Version 1. Available: http://www.searo.who.int/linkfiles/malaria_malariamicroscopymanual.pdf. Accessed: 2012 Sept 26.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

