Abrin immunotoxin: targeted cytotoxicity and intracellular trafficking pathway
- PMID: 23472175
- PMCID: PMC3589266
- DOI: 10.1371/journal.pone.0058304
Abrin immunotoxin: targeted cytotoxicity and intracellular trafficking pathway
Abstract
Background: Immunotherapy is fast emerging as one of the leading modes of treatment of cancer, in combination with chemotherapy and radiation. Use of immunotoxins, proteins bearing a cell-surface receptor-specific antibody conjugated to a toxin, enhances the efficacy of cancer treatment. The toxin Abrin, isolated from the Abrus precatorius plant, is a type II ribosome inactivating protein, has a catalytic efficiency higher than any other toxin belonging to this class of proteins but has not been exploited much for use in targeted therapy.
Methods: Protein synthesis assay using (3)[H] L-leucine incorporation; construction and purification of immunotoxin; study of cell death using flow cytometry; confocal scanning microscopy and sub-cellular fractionation with immunoblot analysis of localization of proteins.
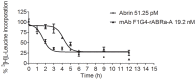
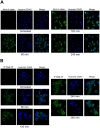
Results: We used the recombinant A chain of abrin to conjugate to antibodies raised against the human gonadotropin releasing hormone receptor. The conjugate inhibited protein synthesis and also induced cell death specifically in cells expressing the receptor. The conjugate exhibited differences in the kinetics of inhibition of protein synthesis, in comparison to abrin, and this was attributed to differences in internalization and trafficking of the conjugate within the cells. Moreover, observations of sequestration of the A chain into the nucleus of cells treated with abrin but not in cells treated with the conjugate reveal a novel pathway for the movement of the conjugate in the cells.
Conclusions: This is one of the first reports on nuclear localization of abrin, a type II RIP. The immunotoxin mAb F1G4-rABRa-A, generated in our laboratory, inhibits protein synthesis specifically on cells expressing the gonadotropin releasing hormone receptor and the pathway of internalization of the protein is distinct from that seen for abrin.
Conflict of interest statement
Figures



Similar articles
-
Inhibition of protein synthesis leading to unfolded protein response is the major event in abrin-mediated apoptosis.Mol Cell Biochem. 2015 May;403(1-2):255-65. doi: 10.1007/s11010-015-2355-9. Epub 2015 Mar 10. Mol Cell Biochem. 2015. PMID: 25753921
-
Selective cytotoxic effects of immunotoxin--monoclonal anti-AFP-abrin-A chain conjugate on several human hepatoma cell lines.Biochem Int. 1990 Oct;22(1):95-102. Biochem Int. 1990. PMID: 1704234
-
Growth suppression of human colorectal carcinoma in nude mice by monoclonal antibody C27-abrin A chain conjugate.Dis Colon Rectum. 1995 Oct;38(10):1067-74. doi: 10.1007/BF02133980. Dis Colon Rectum. 1995. PMID: 7555421
-
Immunotoxin Screening System: A Rapid and Direct Approach to Obtain Functional Antibodies with Internalization Capacities.Toxins (Basel). 2020 Oct 15;12(10):658. doi: 10.3390/toxins12100658. Toxins (Basel). 2020. PMID: 33076544 Free PMC article. Review.
-
Gaps in forensic toxicological analysis: The veiled abrin.Toxicon. 2024 May 6;242:107684. doi: 10.1016/j.toxicon.2024.107684. Epub 2024 Mar 19. Toxicon. 2024. PMID: 38513827 Review.
Cited by
-
The Cytotoxicity of Elderberry Ribosome-Inactivating Proteins Is Not Solely Determined by Their Protein Translation Inhibition Activity.PLoS One. 2015 Jul 6;10(7):e0132389. doi: 10.1371/journal.pone.0132389. eCollection 2015. PLoS One. 2015. PMID: 26148207 Free PMC article.
-
Rapid detection of abrin in foods with an up-converting phosphor technology-based lateral flow assay.Sci Rep. 2016 Oct 5;6:34926. doi: 10.1038/srep34926. Sci Rep. 2016. PMID: 27703269 Free PMC article.
-
Abrus precatorius Poisoning: A Retrospective Study of 112 Patients.Indian J Crit Care Med. 2017 Apr;21(4):224-225. doi: 10.4103/ijccm.IJCCM_320_16. Indian J Crit Care Med. 2017. PMID: 28515607 Free PMC article.
-
Detection of Abrin Holotoxin Using Novel Monoclonal Antibodies.Toxins (Basel). 2017 Nov 28;9(12):386. doi: 10.3390/toxins9120386. Toxins (Basel). 2017. PMID: 29182545 Free PMC article.
-
Optimization of EnBase Fed-Batch Cultivation to Improve Soluble Fraction Ratio of α-Luffin Ribosome Inactivating Protein.Iran J Biotechnol. 2018 Apr 18;16(1):e1482. doi: 10.21859/ijb.1482. eCollection 2018 Apr. Iran J Biotechnol. 2018. PMID: 30555837 Free PMC article.
References
-
- Pirker R (1988) Immunotoxins against solid tumors. J Cancer Res Clin Oncol 114: 385–393. - PubMed
-
- Hertler AA, Frankel AE (1989) Immunotoxins: a clinical review of their use in the treatment of malignancies. J Clin Oncol 7: 1932–1942. - PubMed
-
- Kreitman RJ (2000) Immunotoxins. Expert Opin Pharmacother 1: 1117–1129. - PubMed
-
- Barbieri L, Battelli MG, Stirpe F (1993) Ribosome-inactivating proteins from plants. Biochim Biophys Acta 1154: 237–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

