A calcium-relay mechanism in vertebrate phototransduction
- PMID: 23472635
- PMCID: PMC3689192
- DOI: 10.1021/cn400027z
A calcium-relay mechanism in vertebrate phototransduction
Abstract
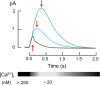
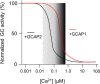
Calcium-signaling in cells requires a fine-tuned system of calcium-transport proteins involving ion channels, exchangers, and ion-pumps but also calcium-sensor proteins and their targets. Thus, control of physiological responses very often depends on incremental changes of the cytoplasmic calcium concentration, which are sensed by calcium-binding proteins and are further transmitted to specific target proteins. This Review will focus on calcium-signaling in vertebrate photoreceptor cells, where recent physiological and biochemical data indicate that a subset of neuronal calcium sensor proteins named guanylate cyclase-activating proteins (GCAPs) operate in a calcium-relay system, namely, to make gradual responses to small changes in calcium. We will further integrate this mechanism in an existing computational model of phototransduction showing that it is consistent and compatible with the dynamics that are characteristic for the precise operation of the phototransduction pathways.
Figures





References
-
- Pugh E. N. Jr., and Lamb T. D. (2000) Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation. In Handbook of Biological Physics (Stavenga D. G., DeGrip W. J., and Pugh E. N. Jr., Eds.), Vol. 4, pp 183–255, Elsevier Science BV: Amsterdam, The Netherlands.
-
- Koch K.-W.; Duda T.; Sharma R. K. (2010) Ca2+-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol. Cell. Biochem. 334, 105–115. - PubMed
-
- Kaupp U. B.; Seifert R. (2002) Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

