Anatomical plasticity of adult brain is titrated by Nogo Receptor 1
- PMID: 23473316
- PMCID: PMC3594793
- DOI: 10.1016/j.neuron.2012.12.027
Anatomical plasticity of adult brain is titrated by Nogo Receptor 1
Erratum in
- Neuron. 2014 Jun 4;82(5):1184-5
Abstract
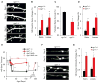
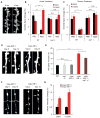
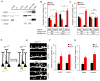
Experience rearranges anatomical connectivity in the brain, but such plasticity is suppressed in adulthood. We examined the turnover of dendritic spines and axonal varicosities in the somatosensory cortex of mice lacking Nogo Receptor 1 (NgR1). Through adolescence, the anatomy and plasticity of ngr1 null mice are indistinguishable from control, but suppression of turnover after age 26 days fails to occur in ngr1-/- mice. Adolescent anatomical plasticity can be restored to 1-year-old mice by conditional deletion of ngr1. Suppression of anatomical dynamics by NgR1 is cell autonomous and is phenocopied by deletion of Nogo-A ligand. Whisker removal deprives the somatosensory cortex of experience-dependent input and reduces dendritic spine turnover in adult ngr1-/- mice to control levels, while an acutely enriched environment increases dendritic spine dynamics in control mice to the level of ngr1-/- mice in a standard environment. Thus, NgR1 determines the low set point for synaptic turnover in adult cerebral cortex.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K, Staiger JF. Expression of c-Fos, ICER, Krox-24 and JunB in the whisker-to-barrel pathway of rats: time course of induction upon whisker stimulation by tactile exploration of an enriched environment. Journal of Chemical Neuroanatomy. 2002;23:187–198. - PubMed
-
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

