Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus
- PMID: 23473324
- PMCID: PMC3595120
- DOI: 10.1016/j.neuron.2012.12.038
Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus
Abstract
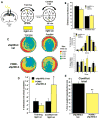
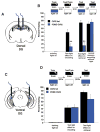
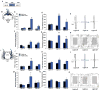
The dentate gyrus (DG), in addition to its role in learning and memory, is increasingly implicated in the pathophysiology of anxiety disorders. Here, we show that, dependent on their position along the dorsoventral axis of the hippocampus, DG granule cells (GCs) control specific features of anxiety and contextual learning. Using optogenetic techniques to either elevate or decrease GC activity, we demonstrate that GCs in the dorsal DG control exploratory drive and encoding, not retrieval, of contextual fear memories. In contrast, elevating the activity of GCs in the ventral DG has no effect on contextual learning but powerfully suppresses innate anxiety. These results suggest that strategies aimed at modulating the excitability of the ventral DG may be beneficial for the treatment of anxiety disorders.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Illuminating hippocampal control of fear memory and anxiety.Neuron. 2013 Mar 6;77(5):803-6. doi: 10.1016/j.neuron.2013.02.017. Neuron. 2013. PMID: 23473311
References
-
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience. 2002;116:884–901. - PubMed
-
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28:273–283. - PubMed
-
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behavioral neuroscience. 1999;113:1170–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

