Fusion peptides promote formation of bilayer cubic phases in lipid dispersions. An x-ray diffraction study
- PMID: 23473485
- PMCID: PMC3870795
- DOI: 10.1016/j.bpj.2012.12.034
Fusion peptides promote formation of bilayer cubic phases in lipid dispersions. An x-ray diffraction study
Abstract
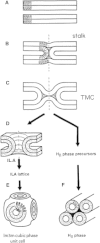
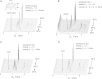
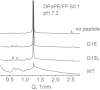
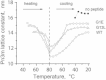
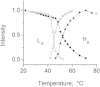
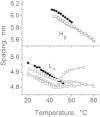
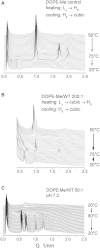
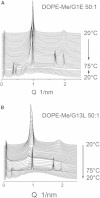
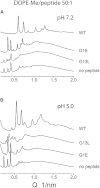
Small angle x-ray diffraction revealed a strong influence of the N-terminal influenza hemagglutinin fusion peptide on the formation of nonlamellar lipid phases. Comparative measurements were made on a series of three peptides, a 20-residue wild-type X-31 influenza virus fusion peptide, GLFGAIAGFIENGWEGMIDG, and its two point-mutant, fusion-incompetent peptides G1E and G13L, in mixtures with hydrated phospholipids, either dipalmitoleoylphosphatidylethanolamine (DPoPE), or monomethylated dioleoyl phosphatidylethanolamine (DOPE-Me), at lipid/peptide molar ratios of 200:1 and 50:1. All three peptides suppressed the HII phase and shifted the L(α)-H(II) transition to higher temperatures, simultaneously promoting formation of inverted bicontinuous cubic phases, Q(II), which becomes inserted between the L(α) and H(II) phases on the temperature scale. Peptide-induced Q(II) had strongly reduced lattice constants in comparison to the Q(II) phases that form in pure lipids. Q(II) formation was favored at the expense of both L(α) and H(II) phases. The wild-type fusion peptide, WT-20, was distinguished from G1E and G13L by the markedly greater magnitude of its effect. WT-20 disordered the L(α) phase and completely abolished the HII phase in DOPE-Me/WT-20 50:1 dispersions, converted the Q(II) phase type from Im3m to Pn3m and reduced the unit cell size from ∼38 nm for the Im3m phase of DOPE-Me dispersions to ∼15 nm for the Pn3m phase in DOPE-Me/WT-20 peptide mixtures. The strong reduction of the cubic phase lattice parameter suggests that the fusion-promoting WT-20 peptide may function by favoring bilayer states of more negative gaussian curvature and promoting fusion along pathways involving Pn3m phase-like fusion pore intermediates rather than pathways involving H(II) phase-like intermediates.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Lentz B.R., Malinin V., Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr. Opin. Struct. Biol. 2000;10:607–615. - PubMed
-
- Nieva J.L., Nir S., Wilschut J. Destabilization and fusion of zwitterionic large unilamellar lipid vesicles induced by a beta-type structure of the HIV-1 fusion peptide. J. Liposome Res. 1998;8:165–182.
-
- Wharton S.A., Martin S.R., Wiley D.C. Membrane fusion by peptide analogues of influenza virus haemagglutinin. J. Gen. Virol. 1988;69:1847–1857. - PubMed
-
- Lear J.D., DeGrado W.F. Membrane binding and conformational properties of peptides representing the NH2 terminus of influenza HA-2. J. Biol. Chem. 1987;262:6500–6505. - PubMed
-
- Murata M., Sugahara Y., Ohnishi S. pH-dependent membrane fusion activity of a synthetic twenty amino acid peptide with the same sequence as that of the hydrophobic segment of influenza virus hemagglutinin. J. Biochem. 1987;102:957–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

