Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial
- PMID: 23473847
- PMCID: PMC3641608
- DOI: 10.1016/S0140-6736(12)62198-9
Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial
Abstract
Background: No trials have investigated routine laboratory monitoring for children with HIV, nor four-drug induction strategies to increase durability of first-line antiretroviral therapy (ART).
Methods: In this open-label parallel-group trial, Ugandan and Zimbabwean children or adolescents with HIV, aged 3 months to 17 years and eligible for ART, were randomly assigned in a factorial design. Randomisation was to either clinically driven monitoring or routine laboratory and clinical monitoring for toxicity (haematology and biochemistry) and efficacy (CD4 cell counts; non-inferiority monitoring randomisation); and simultaneously to standard three-drug or to four-drug induction first-line ART, in three groups: three-drug treatment (non-nucleoside reverse transcriptase inhibitor [NNRTI], lamivudine, abacavir; group A) versus four-drug induction (NNRTI, lamivudine, abacavir, zidovudine; groups B and C), decreasing after week 36 to three-drug NNRTI, lamivudine, plus abacavir (group B) or lamivudine, abacavir, plus zidovudine (group C; superiority ART-strategy randomisation). For patients assigned to routine laboratory monitoring, results were returned every 12 weeks to clinicians; for clinically driven monitoring, toxicity results were only returned for requested clinical reasons or if grade 4. Children switched to second-line ART for WHO stage 3 or 4 events or (routine laboratory monitoring only) age-dependent WHO CD4 criteria. Randomisation used computer-generated sequentially numbered tables incorporated securely within the database. Primary efficacy endpoints were new WHO stage 4 events or death for monitoring and change in CD4 percentage at 72 and 144 weeks for ART-strategy randomisations; the co-primary toxicity endpoint was grade 3 or 4 adverse events. Analysis was by intention to treat. This trial is registered, ISRCTN24791884.
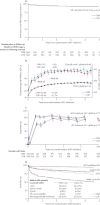
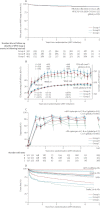
Findings: 1206 children were randomly assigned to clinically driven (n=606) versus routine laboratory monitoring (n=600), and groups A (n=397), B (n=404), and C (n=405). 47 (8%) children on clinically driven monitoring versus 39 (7%) on routine laboratory monitoring had a new WHO stage 4 event or died (hazard ratio [HR] 1·13, 95% CI 0·73-1·73, p=0·59; non-inferiority criterion met). However, in years 2-5, rates were higher in children on clinically driven monitoring (1·3 vs 0·4 per 100 child-years, difference 0·99, 0·37-1·60, p=0·002). One or more grade 3 or 4 adverse events occurred in 283 (47%) children on clinically driven versus 282 (47%) on routine laboratory monitoring (HR 0·98, 0·83-1·16, p=0·83). Mean CD4 percentage change did not differ between ART groups at week 72 (16·5% [SD 8·6] vs 17·1% [8·5] vs 17·3% [8·0], p=0·33) or week 144 (p=0·69), but four-drug groups (B, C) were superior to three-drug group A at week 36 (12·4% [7·2] vs 14·1% [7·1] vs 14·6% [7·3], p<0·0001). Excess grade 3 or 4 events in groups B (one or more events reported by 157 [40%] children in A, 190 [47%] in B; HR [B:A] 1·32, 1·07-1·63) and C (218 [54%] children in C; HR [C:A] 1·58, 1·29-1·94; global p=0·0001) were driven by asymptomatic neutropenia in zidovudine-containing groups (B, C; 86 group A, 133 group B, 184 group C), but resulted in drug substitutions in only zero versus two versus four children, respectively.
Interpretation: NNRTI plus NRTI-based three-drug or four-drug ART can be given across childhood without routine toxicity monitoring; CD4 monitoring provided clinical benefit after the first year on ART, but event rates were very low and long-term survival high, suggesting ART rollout should take priority. CD4 benefits from four-drug induction were not durable, but three-NRTI long-term maintenance was immunologically and clinically similar to NNRTI-based ART and could be valuable during tuberculosis co-treatment.
Funding: UK Medical Research Council, the UK Department for International Development; drugs donated and viral load assays funded by ViiV Healthcare and GlaxoSmithKline.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Monitoring strategies for management of ART in children.Lancet. 2013 Apr 20;381(9875):1343-1344. doi: 10.1016/S0140-6736(13)60284-6. Epub 2013 Mar 7. Lancet. 2013. PMID: 23473849 No abstract available.
References
-
- Gilks CF, Crowley S, Ekpini R. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. - PubMed
-
- Institute for Health Metrics and Evaluation Financing global health 2011: continued growth as MDG deadline approaches. http://www.healthmetricsandevaluation.org/publications/policy-report/fin... (accessed Oct 25, 2012).
-
- UNAIDS . World AIDS Day report 2012. UNAIDS; Geneva: 2012.
-
- Abongomera G, Namata H, Nkhata M, et al. Lablite: baseline mapping survey of decentralised ART service provision in Malawi, Uganda and Zimbabwe. XIX International AIDS Conference; Washington DC, USA; July 22–27, 2012; abstr LBPE572012.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

